Question:
27 The nuclear process that takes place in an atomic bomb or
Last updated: 5/14/2023
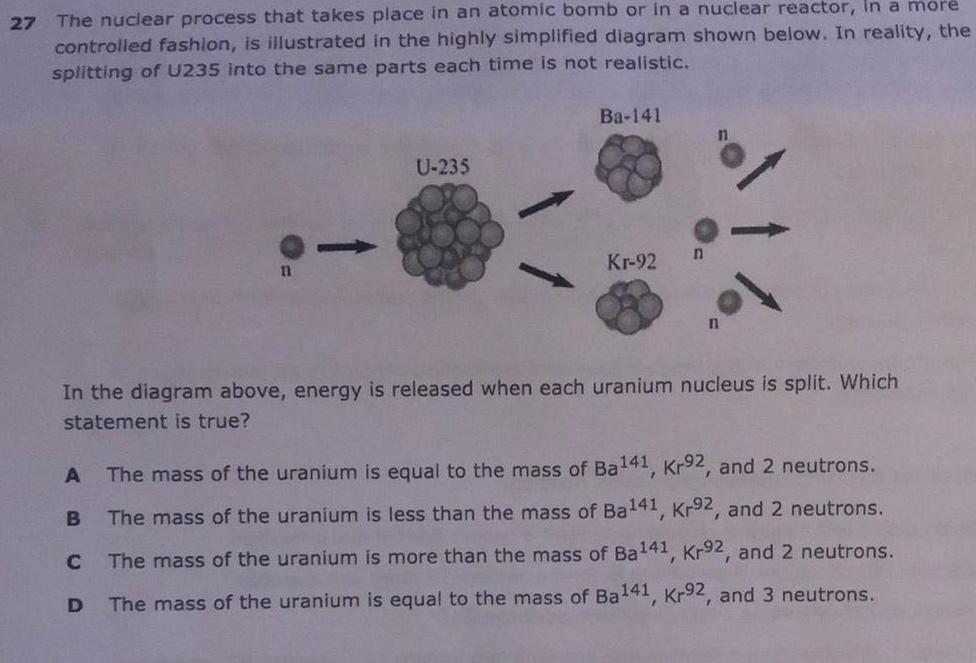
27 The nuclear process that takes place in an atomic bomb or in a nuclear reactor in a more controlled fashion is illustrated in the highly simplified diagram shown below In reality the splitting of U235 into the same parts each time is not realistic A B C 11 D U 235 Ba 141 Kr 92 In the diagram above energy is released when each uranium nucleus is split Which statement is true n The mass of the uranium is equal to the mass of Ba141 Kr92 and 2 neutrons The mass of the uranium is less than the mass of Ba141 Kr92 and 2 neutrons The mass of the uranium is more than the mass of Ba141 Kr92 and 2 neutrons The mass of the uranium is equal to the mass of Ba141 Kr92 and 3 neutrons