Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Electrochemistry9 t respectively 5 0 x 10 13 8 3 x 10 17 Which one of the following salts will precipitate last if AgNO3 solution is added to the solution containing equal moles of NaCl NaBr NaI and Na CrO4 A AgCl B AgBr D AgI C Ag CrO4

Physical Chemistry
GeneralThe molar mass of NH3 is 17 03 g mol and the molar mass of H O is 18 02 g mol Consider the following already balanced chemical equation 4 4 NH3 g 7 O g 4 NO g 6 H O g What is the conversion factor you would use to convert from grams of NH3 to moles of NH3

Physical Chemistry
Nuclear chemistry3 The correct option for free expansion of an ideal gas under adiabatic condition is 1 q 0 AT 0 and w 0 2 q 0 AT 0 and w 0 3 q 0 AT 0 and w 0 4 q 0 AT 0 and w 0

Physical Chemistry
General4 What is the phase difference between two simple TC harmonic motions represented by X A sin ot and X A cos oot 6 a C T 6 T b d F m 5 T 3

Physical Chemistry
ElectrochemistryGiven the standard electrode potentials Pb Pb 0 13 V Sn Sn 0 14 V Zn Zn 0 76 V Mg2 Mg 2 36 V The metal which has the strongest reducing power is Pb Sn Zn

Physical Chemistry
General28 The displacement of a body executing SHM is given by x A sin 2 t 3 The first time from t 0 when the velocity is maximum is b 0 16 sec a 0 33 sec c 0 25 sec d 0 5 sec The displacement of a particle executing SHM is gir

Physical Chemistry
EnergeticsThe molar heat of formation of NH4NO3 s is 367 54 KJ and those of N O g and H 0 e are 81 46 KJ and 285 78 KJ respectively at 27 C and 1 atm pressure What is AH for the reaction NH4NO3 S N O g 2H O l Question Type Single Correct Type 1 2 3 122 56 KJ 449 KJ 122 56 KJ

Physical Chemistry
Chemical kineticser of 156 The rate constants k and k for two different reactions are 1016 e 2000 T and 1015 e 1000 T respectively The temperature at which k K is A tan ol 2 ol L ol C 2000 2 303 1000 2 303 K K B 2000 K D 1000 K

Physical Chemistry
EquilibriumThe value of K for self ionization of formic acid is 10 4 at room temperature What percentage of formic acid is converted to formate ion Given d coo 1 22 g cc A 0 004 B 0 0045 C 0 025 SD 0 037

Physical Chemistry
Atomic StructureIn ncert it is written that nitrogen has higher atomic radius in comparison to oxygen and oxygen has higher atomic radius than fluori ne but in byjus video lessons its taught that oxygen fluorine nitrogen what to follow ple ase help

Physical Chemistry
Generald 1 5 cm 10 A particle is executing SHM Then the graph of velocity as a function of displacement is b Circle d Hyperbola a Straight line c Ellipse

Physical Chemistry
Atomic StructureIf 10 17 of light energy is needed by the interior of human eye to see an object The number of photons of green light 1 550 nm needed to see the object are 1 27 2 28 3 29 4 30 Which of the following statements is false 1 The energy of red photon is more than the energy of violet photon 2 The momentum of photon is inversely proportional to its wave length 3 The energy of a photon is inversely proportional to its wave length

Physical Chemistry
Chemical BondingEach NH3 molecule has six other NH3 molecules as nearest neighbors in solid state AH Of sublimation of solid NH3 at its m p is 30 8 kJ mol and in the absence of H bonding estimate A of sublimation is 14 4 kJ mol The strength of H bond in solid NH3 is A 5 47 kJ mol B 10 93 kJ mol C 16 40 kJ mol D 16 40 kJ mol 1

Physical Chemistry
Atomic StructureHow many photons of light having a wavelength of 5000 are necessary to provide 1 joule of energy 1 2 8 10 8 photons 10 7 photons 10 4 photons 3 2 5 1018 photons 2 2 5 4 2 6 RT Bohr Model The ionization energy of Het is 19 6 x 10 18 J atom The energy of the first stationary state of Li 2 will be 1 84 2 x 10 18 J atom 2 44 10 x 10 18 J atom

Physical Chemistry
GeneralThe observed rotation of a sample subjected to polarimetry is 0 0 Which statement is accurate about the identity of this sample The sample is a mess compound All of the statements are accurate The sample is a racemic mixture The sample is achiral

Physical Chemistry
Atomic StructureWhich of the following statements is false 1 The energy of red photon is more than the energy of violet photon 2 The momentum of photon is inversely proportional to its wave length 3 The energy of a photon is inversely proportional to its wave length 4 The particle nature of electromagnetic radiations is able to explain the photoelectric effect Light of wavelength I falls on metal having work function hc l Photoelectric effect will take place onl 21 COLL 21

Physical Chemistry
Solid state22 If a body is executing simple harmonic motion and in 3 times the amplitude from 2 current displacements is its mean position then the ratio between potential energ and kinetic energy a 3 2 c 3 1 C is b 2 3 d 3 1

Physical Chemistry
GeneralFrom the following energy levels of hydrogen atom the values of E and E3 in J are respectively I E E3 E 0 545 10 8 J E 2 18 10 18 J TS EAMCET Engg 2019 0 242x10 A 1 0 242 10 19 O A 1 18

Physical Chemistry
Atomic StructureAn atom has a mass of 0 02 kg and uncertainty in its velocity is 9 218 x 10 6 m s then uncertainty in position is h 6 626 10 34 Js A 2 86 1028 m C 1 5 10 27 m B 2 86 10 32 cm D 3 9 10 0 m

Physical Chemistry
GeneralIs also of R configuration Is also of S configuration Rotates the propagation plane of polarized light in clockwise fashion Is also of configuration

Physical Chemistry
GeneralWhat is it that is rotated in a polarimetry experiment The priorities around asymmetrically substituted tetrahedral carbons The chiral molecules The chiral centers of molecules Plane polarized light The propagation plane of light

Physical Chemistry
General1 6 4 x 1024 2 7 2 x 1024 3 2 1 x 101 4 3 3 101 A photon in X region is more energetic than in the visible region X is 2 UV 3 Microwave 4 Radio wave 1 IR Electromagnetic radiations of wavelength 242 nm is just sufficient to ionise Sodium atom Then the ionisatio energy of Sodium in KJ mole is 1 494 65 2 400 3 247 4 600

Physical Chemistry
GeneralWhich of the following is not permissible arrangement of electrons is an atom A n 3 1 2 m 2 s 1 2 B n 4 1 0 m 0 s 1 2 C n 5 1 3 1 3 m 0 s 1 2 2 m 3 s 1 2 D n

Physical Chemistry
Nuclear chemistryb Frequency of this light is 5 x 10 4 S c Energy of photon is approximately 2 07 eV d Both b and c 4 Photon having wavelength 310 nm is used to break bond of A molecule having bond energy 288 kJ m then the percentage of energy of photon converted to is 1 eV 96 kJ mol a 25 b 50 T1 c 75 d 80
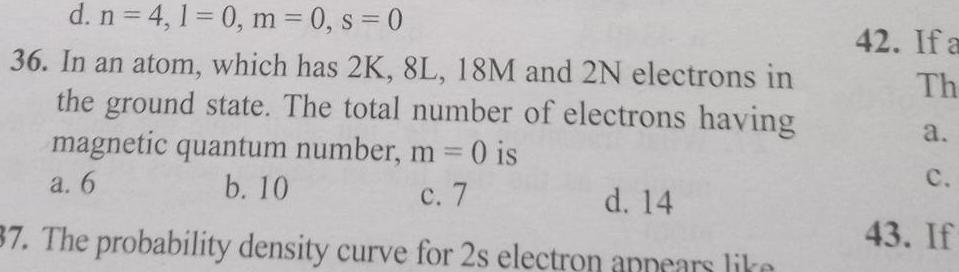
Physical Chemistry
Generald n 4 1 0 m 0 s 0 36 In an atom which has 2K 8L 18M and 2N electrons in the ground state The total number of electrons having magnetic quantum number m 0 is a 6 b 10 c 7 d 14 37 The probability density curve for 2s electron appears like 42 If a Th a C 43 If

Physical Chemistry
Generald 1s 7 Two simple harmonic motions A and B are giver respectively by the following equations y a sin cot TC y a sin oot Phase difference between the waves is a C EN R M TC 2 T 3r 6 3 b T 6 d Zero

Physical Chemistry
GeneralH3PO4 is a tribasic acid and one of its salt is NaH PO4 What volume of 1M NaOH solution should be added t 12 g NaH PO4 to convert it into Na3PO4 at wt of P 31 100 mL 200 mL CORRECT ANSWER 80 mL 300 ml

Physical Chemistry
General34 Suppose that a hypothetical H like atom gives a red green blue and violet line in spectrum Which jump according to figure would give off the violet spectral line n 4 a 3 1 c 4 1 n 3 n 2 n 1 b 2 1 d 3 2

Physical Chemistry
Equilibrium162 The reaction 2A g B g 3C g D g is start with the concentrations of A and B both at an initial value of 1 00 M When equilibrium is reached the concentration of D is measured and found to be 0 25 M The value for the equilibrium constant for this reaction is given by the expression A 0 75 0 25 0 50 0 75 B 0 75 0 25 0 50 0 25 C 0 75 0 25 0 75 0 25 D 0 75 0 25 1 00 1 00

Physical Chemistry
Atomic StructureCalculate the energy of a photon of sodium light of wave length 5 826 1 3 41 10 J 2 1 3 41 10 1 J 3 3 41 10 15 J 10 16 m in Jules Calculate the frequency of a photon of wavelength 4000 1 7 5 10 4 S 1 2 7 5 10 16 S 1 3 8 10 4 S 1 Calculate the wavelength of a photon having an energy of 2 electron volt 4 1 3 41 10 2 J 4 6 5 10 15 S 1

Physical Chemistry
Atomic Structurea 2 2 x 10 19J c 4 x 10 20J b 2 10 J d 2 10 20J 1 What is the maximum number of electrons which can accomodated in principal quantum number 4 a 16 b 18 c 22 d 32

Physical Chemistry
GeneralOne g of hydrogen is found to combine with 80 g of bromine One g of calcium valency 2 combines with 4 g of bromine The equivalent weight of calcium is 10 20 CORRECT ANSWER

Physical Chemistry
Chemical kineticsis isost BaCl IC1 4 S n a first order reaction AB if h constant and initial concentration of the reactant A is 0 5 M then the half life is In 2 k 3 log2 2 0 693 0 5k log2 k 0 5 78 and PB are the vapour pressure of pure liquid pents A and B respectively of an ideal A the total pressure of on If x represents the mole

Physical Chemistry
General12 A particle executes SHM its time period is 16 sec If it passes through the centre of oscillation then its velocity is 2 m s at time 2 sec The amplitude will be V

Physical Chemistry
General3 Series combin m 2 K 4 T 2n K K 20 When the displacement of a particle executing simple harmonic motion is half its amplitude the ratio of its kinetic energy to potential energy is a 1 3 b 2 1 c 3 1 d 1 2

Physical Chemistry
Energetics7 For the reaction 298 K 24 B C AH 100 kcal and AS 0 050 kcal K If AH and AS are assumed to be constant over the temperature range above what temperature will the reaction become spontaneous 1 1000 K 2 1500 K 3 2000 K 4 2500 K

Physical Chemistry
Atomic Structureonly 6 In which transition one quantum of e emitted a n 4 n 2 b n 3 n 1 d All of these c n 2 n 1 7 Last line of Lyman series for H

Physical Chemistry
SolutionsConsider the following diagram 1 Solution SPM Solvent Container 1 Container 2 SPM is the semi permeable membrane P and P are the pressure applied Identify the incorrect statement If P P then liquid will flow from container 2 to container 1 To stop the osmosis P must be equal to osmotic pressure To carry out osmosis P must be lesser than P If P is greater than P then reverse osmosis will occur

Physical Chemistry
Chemical BondingWhich of the following is the correct curve for an orbital having set of quantum numbers n 1 m as 2 1 0 respectively showing variation in wave function radial component with respect to distance from the nucleus

Physical Chemistry
Atomic Structure18 If AE is the energy emitted in eV when an electronic transition occurs from higher energy level to lower energy level in H atom the of the line produced is approximately equal to b a C 12375 A AE 13600 A AE d 19800 A AE 21800 A AE

Physical Chemistry
Electrochemistry0 Corrosion of iron is essentially an electrochemical phenomenon where the cell reactions are a Fe is oxidised to Fe2 and dissolved oxygen in water is reduced to OH 8 b Fe is oxidised to Fe3 and H O is reduced to 02 c Fe is oxidised to Fe2 and H O is reduced to 0 d Fe is oxidised to Fe and H O is reduced to 0

Physical Chemistry
Atomic Structurea 2 4 x 1025 Hz c 1 6 x 1015 Hz 5 The total number of orbitals in the principal shell of He Rhc where R is Rydberg 4 b 2 4 x 1016 Hz d 8 x 10 5 Hz that has energy equal to constant a 4 b 8 c 16 d 32

Physical Chemistry
ElectrochemistryDesign electrochemical cells in which each of the following reactions occurs 3 a Ce4 aq Fe aq Ce aq Fe aq b Ag aq Cl aq AgCl s c HgO s H g Hg 1 H O 1 In each case write the representation of the cell and the reactions at the two electrodes

Physical Chemistry
Generalb 0 69 m d 0 61 m c 0 41 m 14 A body executing SHM has velocity 10 cm s and 7 cm s when its displacements from the mean position are 3 cm and 4 cm respectively length of path a 10 cm b 4 8 cm c 4 cm d 11 36 cm J 2 KE 3 TE

Physical Chemistry
ElectrochemistryFor the following galvanic cell Ell 0 03 V at 25 C cell Pt s H g 1 atm H aq 1 M Ag aq 1 M Ag s Calculate the value of AG Ag aq Given 1 F 96500 C moi 2895 kl 28 95 kJ

Physical Chemistry
General2 Simple pendulum T 2x 32 The period of oscillation of a simple pendulum of constant length at surface of the earth is T Its time period inside mine will be a Increases c No change 33 If the b Decreases d None

Physical Chemistry
GeneralQuestion No 35 40 The equation zo RT is applicable to AO Multilayer film at surface BO Monolayer film at surface CO None of these DO Calculate osmotic pressure

Physical Chemistry
Generala 6 b 10 c 7 d 14 37 The probability density curve for 2s electron appears like R r th h r C a R2 R b R2 d r

Physical Chemistry
Gaseous and liquid states8 Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively The molecular mass of A is 49 u Molecular mass of B will be a 50 00 u c 6 50 u b 12 25 u d 25 00 u

Physical Chemistry
Energetics30 For given following equations and AH values determine 90 the enthalpy of reaction at 298 K for the reaction C H g 6F g 2CF g 4HF g H g F g 2HF g AH 537 kJ C s 2F g CF 9 AHS 680 kJ 2C s 2H g C H g AH 52 kJ 1 1165 2 2486 3 1165 HAFT