Chemical kinetics Questions and Answers

Physical Chemistry
Chemical kineticser of 156 The rate constants k and k for two different reactions are 1016 e 2000 T and 1015 e 1000 T respectively The temperature at which k K is A tan ol 2 ol L ol C 2000 2 303 1000 2 303 K K B 2000 K D 1000 K

Physical Chemistry
Chemical kineticsis isost BaCl IC1 4 S n a first order reaction AB if h constant and initial concentration of the reactant A is 0 5 M then the half life is In 2 k 3 log2 2 0 693 0 5k log2 k 0 5 78 and PB are the vapour pressure of pure liquid pents A and B respectively of an ideal A the total pressure of on If x represents the mole

Physical Chemistry
Chemical kinetics55 Half life period of a first order reaction is 1386 seconds the specific rate constant of the reaction is A 5 0 x 10 2 s 1 C 0 5 x 10 S 1 B 5 0 x 10 3 s 1 D 0 5 x 10 3 S 1

Physical Chemistry
Chemical kineticsIn reversible reaction AB the initial concentration of A and B are a and b in moles per litre and the equilibrium concentrations are a x and b x respectively express x in terms of K K a and b K a K b K K K a K b K K b K a K b K K d K a K b K K

Physical Chemistry
Chemical kineticsIn a zero order reaction for every 10 C rise of temperature the rate is doubled If the temperature is increased from 10 C to 100 C rate of reaction will becomes b 512 times d 128 times a 256 times c 64 times

Physical Chemistry
Chemical kineticsThe value of rate constant for a first order is 2 303 10 sec What will be the time required 1 to reduce the concentration to concentration a 100 sec c 2303 sec 10 th of the initial b 10 sec d 23 03 sec

Physical Chemistry
Chemical kineticsConsider the reaction 2N O 4NO 0 In the reaction NO is being formed at the rate of 0 0125 mol Ls What is the rate of reaction at this time a 0 0018 mol Ls b 0 0031 mol L s c 0 0041 mol Ls d 0 050 mol L s

Physical Chemistry
Chemical kineticsWhen the following reaction come to equilibrium N g 2H g NH g K 7 4 10 the equilibrium mixture contains a Mostly products b Mostly reactants c It can not be predicted d Sometimes reactants and sometimes products

Physical Chemistry
Chemical kinetics9 In Arrhenius equation k Ae RI A may not be termed as rate constant 1 When 100 reactant will convert into the product 2 When the temperature becomes infinite 3 When the fraction of molecule crossing over the energy barrier becomes unity 4 At very low temperature

Physical Chemistry
Chemical kinetics46 For the chemical process energies are plotted in graph Energy C a e fb Progress of reaction Which of the following is correct 1 It is the exothermic reaction AH b a 2 Threshold energy e a c 3 E Ea b A All of these

Physical Chemistry
Chemical kineticsThe pyknometric density of sodium chloride crystal is 2 165 x 10 kg m 3 while its X ray density is 2 178 10 kgm The fraction of unoccupied sites in sodium chloride crystal is X 2 5 96 x 10 4 5 96 x 10 3 1 5 96 3 5 96 10 1

Physical Chemistry
Chemical kineticsIn a reaction 2X Y the concentration of X decreases from 3 0 moles litre to 2 0 moles litre in 5 minutes The rate of reaction is b 5 mol L min a 0 1 mol L min c 1 mol L min d 0 5 mol L min

Physical Chemistry
Chemical kinetics2HI g H g L g The equilibrium constant of the above reaction is 6 4 300 K If 0 25 mole each of H and I are added to th system the equilibrium constant will be a 0 8 b 3 2 c 1 6 d 6 4

Physical Chemistry
Chemical kineticsCalculate the rate of the reaction CH3CO 0 H O 2CH3COH 1 if its rate constant is equal to 1 0 0454 min the concentration of acetic anhydride is equal to 0 45 mol l The reaction is first order with respect to acetic anhydride zero order for water

Physical Chemistry
Chemical kinetics3 The half life of a substance is the length of time for an unstable element to spontaneously decay to one half of its original amount Plutonium 239 has a half life of 24 years The formula for half life is as follows M 100 0 5 Determine how long it would take for the sample to decay to only 5 0 g 31

Physical Chemistry
Chemical kinetics33 The result of three experiments carried out for etermination of differential rate of reaction Cl2 g 2NO g i Mention differential rate law for reaction ii Mention order of reaction iii Find value of specific rate constant Experiment number 2NOCI are as follows then 1 2 3 Initial concentration mole litre Cl 0 06 0 06 0 02 NO 0 03 0 08 0 08 S L Section D 8 4 marks Initial rate of reaction d Cl dt mole litre sec 0 0054 0 0384 0 0128

Physical Chemistry
Chemical kineticswas observed that in a reaction on doubling the concentration of reactant rate remains unaffected The order of the reactio A

Physical Chemistry
Chemical kineticsQ 36 At 300 K the rate of decomposition of a gaseous compound initially at a pressure of 20 kPa was 13 5 Pas when 10 had reacted and 0 5 Pas when 70 had reacted Then Calculate order of reaction is Chestik emistarpel

Physical Chemistry
Chemical kineticsIn a reaction A B Product rate is doubled when the concentration of B is doubled and rate increases by a factor of 8 when the concentrations of both the reactants A and B are doubled rate law for the reaction can be written as AIRMT Prelims 20121
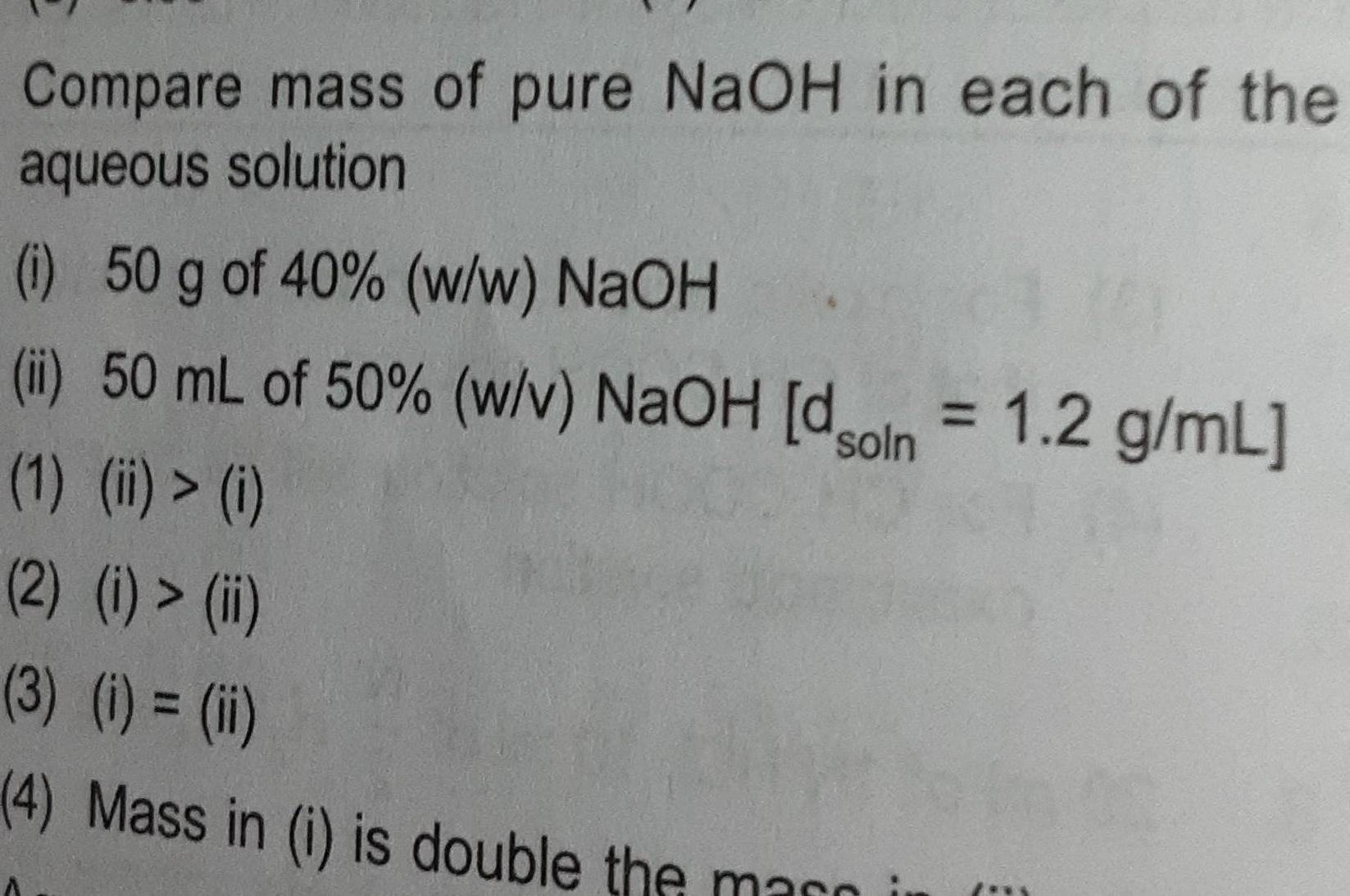
Physical Chemistry
Chemical kineticsCompare mass of pure NaOH in each of the aqueous solution i 50 g of 40 w w NaOH ii 50 mL of 50 w v NaOH dson 1 2 g mL 1 ii i 2 i ii 3 i ii 4 Mass in i is double the marni

Physical Chemistry
Chemical kinetics47 Minimum amount of Ag CO s required to produce 47 Ag CO s Cuide de fac S T P CO sufficient oxygen for the complete combustion of C H which produces 11 2 ltr of CO at S T P after combustion is Ag 108 Ag CO3 s 2Ag s CO g 1 2O g C H 5 20 2CO H O 1 276 g 11 2 2 345 g 227 Ag cu 3 690 g 4 1380 g 100 11 2 Ag 108 Ag CO s 2Ag s CO g 1 2O g C H 5 20 2CO H O 1 276 g 2 345 g 3 690 g 4 1380 g

Physical Chemistry
Chemical kineticsThe half life of a substance in a certain enzyme catalysed reaction is 138 s The time required fo the concentration of the substance to fall from 1 28 mg L 1 to 0 04 mg L is 1 690 s AIPMT Mains 201 2 276 s

Physical Chemistry
Chemical kineticsFor a reaction 2R P concentration of reactant was measured as a function of time Calculate half life of the reactant at a concentration of 0 6 M in term of min R Molarity 0 5 10 time minutes 3 0 7 0 2 16 3

Physical Chemistry
Chemical kinetics48 In three different reactions involving a single reactant in each case a plot of rate of the reaction on the y axis versus concentration of the reactant on the x axis gives three different curves shown below 1 C 8 dc ii dt 4 C iii dc dt C The possible orders of the reactions i ii and iii respectively are

Physical Chemistry
Chemical kinetics5 For the reaction CH CI OH aq aq The kinetic data are as given below CH CI OH CH OH CT aq a 10 18 b 10 15 c 10 5 aq d CH OH dt M min 3 0 2 0 1 2 10 0 4 0 1 4 10 0 4 0 2 8 10 X If K for the above reaction is 1 10 4 then the specific reaction rate M min for the replacement of OH group of methanol by Cl atom is

Physical Chemistry
Chemical kinetics1 2 32 Four vessels 1 2 3 and 4 contain respectively 10 mol atom t1 2 10 hours Imol atom t 5 hours 5 mol atom t 2 2 hour and 2 mol atom t 2 1 hour of different radioactive nuclides In the beginning the maximum radioactivity would be exhibited by the vessel a 4 b 3 c 2 d 1

Physical Chemistry
Chemical kinetics4 temperature pressure and catalyst 83 AH of water is 285 5 kJ mol if enthalpy of neutralisation of monoacidic strong base is 57 3 kJ mol AH of OH ion will be 84 1 228 2 kJ mol 3 114 5 kJ mol Which of the following compound has the 2 228 5 kJ mol 4 114 5 kJ mol

Physical Chemistry
Chemical kinetics30 min Time A 10 w w solution of cane sugar has undergone partial inversion according to the reacti Sucrose water glucose fructose The boiling point of the solution is 100 7 C What fraction of sugar has inverted K H O 1 8 K kg mol

Physical Chemistry
Chemical kineticsIn first order reaction A D the ratio of a a x was found to be 8 after 60 minutes If the concentration is 0 1M then the rate of reaction in moles of A reacted per minute is 2 226 10 3 mol liter 1 min 1 3 466x10 3 mol liter 1 min 1 4 455x10 3 mol liter min 1 5 532x10 3 mol liter 1 min

Physical Chemistry
Chemical kinetics75 The plot of In Kea versus versus inverse of a reaction is shown as eq ba In K T K K K L T T2 The reaction should be 1 Endothermic 2 With no change in enthalpy 3 Exothermic 4 Always spontaneous 1 In ut

Physical Chemistry
Chemical kineticsThe nucleus of the element having atomic number 25 and atomic weight 55 will contain CPMT 1986 MP PMT 1987 a 25 protons and 30 neutrons b 25 neutrons and 30 protons c 55 protons

Physical Chemistry
Chemical kineticsInversion of a sugar follows first order rate equation which can be followed by noting the change in rotation of the plane of polarization of light in a polarimeter If Too Tt To are the rotations at t t t and t 0 then first order reaction can be written as a k log k log log log b c k d k rt to TO Tx 70 7x TI TO Too TO Too Tt Too Tt

Physical Chemistry
Chemical kineticsThe following results were obtained during kinetic studies of the reaction 2A B Products The time in minutes required to consume half of A is 1 5 1 10 Exp 1 2 3 A in mole L 0 10 0 10 0 20 B in mol L 0 20 0 25 0 30 Initial rate of reaction M min 6 93 x 10 6 93 x 10 1 386x102

Physical Chemistry
Chemical kinetics10 moles of a active radio isotope M g are disintegrating at 300 K in a cylinder enclosed with a frictionless piston as given below A 12 4M g 2N g a particles Z 6 The half life of the radioactive sample is 69 3 hours The a particles emitted forms He g by trapping electrons in the cylinder As He g forms piston moves up against constant external pressure of 1 atm The work done by the gas in cal by the end of initial one hour is assume rate remains almost constant during this period Given R 2 Cal mol K In 2 0 693 Report your answer by dividing it by 30

Physical Chemistry
Chemical kineticsIncreasing the temperature O of 100 g of water through 100 C O O O Increasing the temperature of 1 g of water through 100 C Increasing the temperature of 500 g of water through 100 C Increasing the temperature of 10 g of water through 100 C

Physical Chemistry
Chemical kineticsreater eater aller 46 For the reaction AnB the concentration of A decreases from 0 06 to 0 03 mol L 1 and that of B rises from 0 to 0 06 mol L 1 at equilibrium The values of n and the equilibrium constant for the reaction respectively are a 2 and 0 12 b 2 and 1 2 c 3 and 0 12 d 3 and 1 2 ation of othyl methyl ketone with Cl 477 MI

Physical Chemistry
Chemical kinetics62 4 None of these A gaseous reaction 2A g B g 5C g shows increase in pressure from 80 mm to 100 mm in 5 minutes The rate of disappearance of A is 1 4 mm min 3 1 mm min TE 2 8 mm min 4 2 mm min

Physical Chemistry
Chemical kineticsQuestion The half life period of a first order reactionis 5 minutes at A 0 1 M If the conc of A is doubled then half life becomes Half Twice Unaltered Three times

Physical Chemistry
Chemical kinetics61 The reaction of formation of phosgene from 6 CO and Cl is CO CI COCI The proposed mechanism is 1 CI 2Cl fast ii CI CO COCI fast iii COCI CI COCI Cl slow Find the correct expression of rate law 1 k 1 r k x 2 r k x k 3 r k x 4 None of these CO C1 1 2 CO CI CO C1 1 2 CI 3 6

Physical Chemistry
Chemical kineticsion C 1 A 1 a d 2 b c 3 a b d 4 a b c d 59 The half life period of a second order reaction is 30 minutes If the initial conc is 0 1 M the rate constant will be 1 0 666 mol L min 2 0 333 mol L min 3 0 444 mol L min 4 0 555 mol L min

Physical Chemistry
Chemical kinetics5 In a first order reaction the concentration of the reactant decreases from 0 8 M to 0 4 M in 15 minutes The time taken for the concentration to change from 0 1 M to 0 025 M is 1 60 minutes 3 7 5 minutes 2 15 minutes 4 30 minutes The rate for a first order ropotion C

Physical Chemistry
Chemical kineticsThe following mechanism has been suggested for the reaction H O 2H 21 1 2H O 1 H O 1 2 OH H H O 3 HOI H 1 1 H O Identify the rate law that s consistent with the mechanism A Ratek H O 1 B Rate k HO H C Rate klOH H D Rate k HO HD E Rate k H O HP OD OA DE HOI OH Slow Fast Fast

Physical Chemistry
Chemical kinetics3 Match each of the diatomic molecules ions in Column I with its property properties in Column II Column I Column II JEE 2009 A B P Paramagnetic Q undergoes oxidation R Undergoes reduction S Bond order 2 T Mixing of s and p orbitals LIEE 20001

Physical Chemistry
Chemical kineticshydrogen released vs time for A graph of vol the reaction between zinc and dil HCl is given in figure On the basis of this mark the correct option V5 V3 V V 0 20 30 40 50 a Average rate upto 40 seconds is b Average rate upto 40 seconds is V c Average rate upto 40 seconds is d Average rate upto 40 seconds is c V5 V 50 30 10 Consider the graph given in Q 9 Which of the following options does not show instantaneous rate of reaction at 40th second a V3 V 2 40 30 V3 V 40 V3 V 40 30 b d 3 40 V4 V 50 30 V3 V 40 20 V3 V 40 20

Physical Chemistry
Chemical kineticsc The rate of a first order reaction is 0 04 mol s at 10 seconds and 0 03 mol s at 20 seconds after initiation of the reaction The half life period of the reaction is 2016 a 24 1s b 34 1 s d 54 1s c 44 1s fa catalyst during a chemical an P At

Physical Chemistry
Chemical kinetics8 pH for the solution of salt undergoing anionic hydrolysis say CH3COONa is given by 1 pH 1 2 pKw pK log C a 2 pH 1 2 pKw pKa log C W 3 pH 1 2 pKw pk log C 4 None of these

Physical Chemistry
Chemical kinetics12 The rate constant of a certain reaction is given by log K B C log T Then activation energy of reaction at A T 300 K is B 1 Clog T T 3 Clog T 2 2 303 B CT R 4 B CTR

Physical Chemistry
Chemical kineticsFor an ionic compound lattice energy magnitude only is 30 kJ and combined hydration energy of ions magnitude only is 40 kJ Enthalpy of solution of the compound is DA 70 kJ OB 10 kl Oc 10 kl D 70 kJ

Physical Chemistry
Chemical kineticsreaction The reaction X 2Y Z N occurs by the follow mechanism i X Y M very rapid equilibrium ii M ZO slow iii O Y N very fast What is the rate law for this reaction 1 Rate k Z 3 Rate k N The rate of the reaction 2 Rate k X Y z 4 Rate k X

Physical Chemistry
Chemical kineticsConsider a first order decomposition process 3 4342 A plot of concentration of A3 and A2 versus time is show below At time t4 percentage of reactant decomposed is Concentration tA A A3