Question:
Calculate the mass in grams of Fe2S3 (iron III sulfide) that
Last updated: 8/4/2022
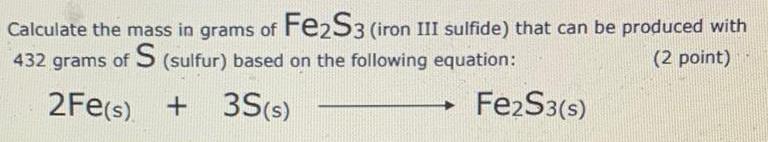
Calculate the mass in grams of Fe2S3 (iron III sulfide) that can be produced with 432 grams of S (sulfur) based on the following equation: (2 point) 2Fe(s) + 3S (s) → Fe2S3(s)