Question:
The electrochemical cell shown below is a concentration cell
Last updated: 6/30/2023
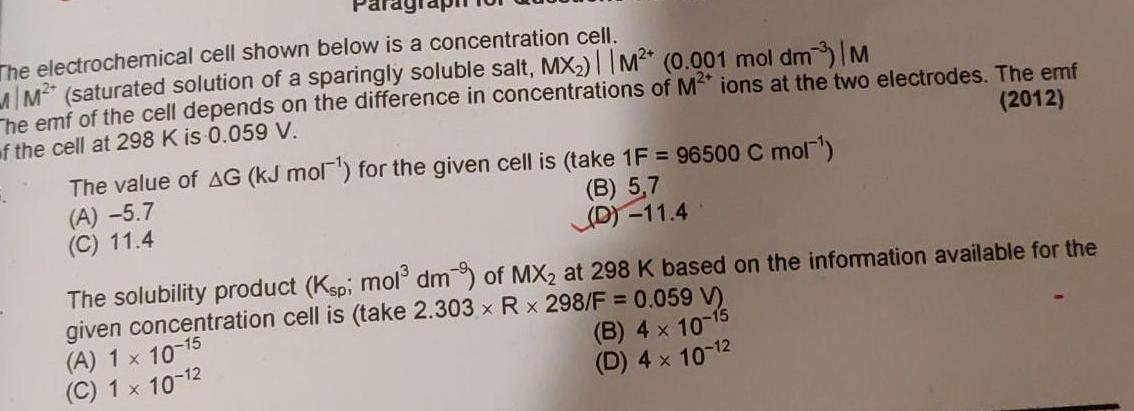
The electrochemical cell shown below is a concentration cell MM saturated solution of a sparingly soluble salt MX M 0 001 mol dm3 M he emf of the cell depends on the difference in concentrations of M ions at the two electrodes The emf of the cell at 298 K is 0 059 V 2012 The value of AG kJ mol for the given cell is take 1F 96500 C mol B 5 7 D 11 4 A 5 7 C 11 4 The solubility product Ksp mol dm of MX at 298 K based on the information available for the given concentration cell is take 2 303 x R x 298 F 0 059 V B 4 x 10 15 D 4 x 10 A 1 x 10 15 C 1 x 10 12