S Block - Group 1 Questions and Answers

Inorganic Chemistry
S Block - Group 1Homeostasis is the ability of the body to O ignore external stimuli to remain in a state of rest O prevent the external environment from changing O prevent the internal environment from changing O quickly restore changed conditions to normal O prevent excessive blood loss

Inorganic Chemistry
S Block - Group 1Select all statements describing metals forms ionic compounds with nonmetals bad conductor of electricity good conductor of heat found on the left side of the periodic table tends to gain electrons

Inorganic Chemistry
S Block - Group 1NiO 2Cu 4H Ni2 2Cu 2H O the above reaction the oxidation state of nickel changes from w many electrons are transferred in the reaction to

Inorganic Chemistry
S Block - Group 14 Calculate the following A How many moles of HCI are present in 85 5 mL of a 0 160 M HCl solution B Determine the volume of 0 230 M NaOH required to neutralize the HCI solution

Inorganic Chemistry
S Block - Group 1c How many moles of NaOH are required for the titration 203 d Calculate the Molarity of NaOH solution Molarity of NaOH moles NaOH volume of NaOH added in L

Inorganic Chemistry
S Block - Group 1Physiology is the study of O the structure of the body the normal function of living organisms O the tissues and organs of the body at the microscopic level the facial features as an indication of personality O growth and reproduction

Inorganic Chemistry
S Block - Group 1What is the bond order of the carbon oxygen bond in COF2 1 1 5 2 2 5 3 0

Inorganic Chemistry
S Block - Group 1Label all bonds on the sketch of the structure Drag the appropriate labels to their respective targets Labels can be used once more than once or not at all o N sp H s o N p H s N sp N sp o N p N p T N p N p T N sp N sp Lone pair in N sp H 1 H H H Reset

Inorganic Chemistry
S Block - Group 12 This element is select 8 Identify the element from the atom shown What are the atomic number neutron number and number of this isotope The atomic number of this isotope is The neutron number of this isotope is The mass number of this isotope is Check my work electrons Expanded view

Inorganic Chemistry
S Block - Group 1Complete the following table for the designated atoms Isotope Symbol Number of Protons Number of Neutrons Number of Electra 56 Mn 25 23 Na 11 12 Check my work 11 6

Inorganic Chemistry
S Block - Group 16 This structure can be used by pathogenic bacteria to attach to epithelial cells A Cell membrane B Cell wall C Endospores D Fimbriae E Ribosomes 7 Which of the following products are made during glycolysis A ATP B NADH C Pyruvic acid D A and B E All of the above

Inorganic Chemistry
S Block - Group 14 Which of the following taxonomic groups is the largest contains the most organisms A Class B Domain C Family D Kingdom E Order 5 One of the important characteristics of active transport is A It can only move substances from high to low concentration B It can only move substances from outside to in C It only occurs in eukaryotes not prokaryotes D It requires energy E It results in antibody production

Inorganic Chemistry
S Block - Group 1Ample of nitrogen occupies a occupy at 05 degrees Ce Boyle s Law Char Law Gay Lu has a pressure of 0 370 am at 500 C What is the pressure at standard temperatur Boyle s Lawb Charles and C Gay Lussss Lawd ak 3 Cakulate the decrease in tempera A 1953 Kelvin b 1956 Kave me of 250m at 25 degrees Celsus What volume w ve this problem low 4A400L tank of ammonis has a pressure of 12 7 AP Calculate the volume of the ammonia if its pressure is changed to 8 4 APa while its temperature remains constant 62 0 Sters b 03 0 aters 00 5 ers d 62 5 Ster 6 How many neutrons does Radon have a b 80 e 136 SA sample of Oxygen gas occupies a volume of 250 ml at 740 for pressure What volume will it occupy at 600 torr pressure Which law would you use to solve this problem A Boyle s Low b Charles Law c Gay Lussacs Law d B and C when 0 00 at 20 0 Ce compressed to 4 00 L 106 Refox3 Kevin 8 How many protons does Radon have b 86 c 136 a 222 7 How many electrons does Radon have a 222 b 86 c 136 9 How many energy levels does Radon have a 2 c 6 b 4 d 8 d 308 d 308 The most stable group of elements is the a halogens d 308 10 The vertical direction up and down on a periodic table is referred to as a periods b columns c lines d towers 11 The horizontal direction side to side on a periodic table is referred to as a periods b columns c lines d towers 12 Which of the following elements can be found in group 2 period 77 a Radon b Francium c Radium d Krypton b metalloids c alkaline earth metals d noble gasses the mass in grams of one mole of a substance is best defined as the Percent of mole b Molar mass c molar balance d None of these ich answer best defines the molar mass of sodium carbonate Na2CO3 108 2 g mole b 106 0 g mole c 160 0 g mole d 106 3 g mol

Inorganic Chemistry
S Block - Group 136 The configuration that is desired by the atoms is that of alan a noble gas b halogen c earth metal d alkaline metal 37 Draw the Bohr model for Sodium 4 points For questions 38 43 complete the following information based on the element Sodium 38 How many protons 39 How many neutrons 40 How many electrons 41 How many valence electrons 42 What is the atomic number 43 What is the atomic mass 44 If an atom has a mass of 11 and 5 electrons its atomic number must be a 11 b 16 C 10 d 5 45 Covalent compounds form bonds by a donating b sharing c taking d None of these 46 The following elements are involved in covalent bonding a metal to metal b metal to nonmetal c nonmetal to nonmetal d halogen to noble gas 47 A molecule of NH3 contains electrons lonic bonds only b covalent bonds only c ionic and covalent bonds d neither ionic or covalent 48 In ionic bonding all metals an electron to become stable a share b accept c receive d donate 49 In ionic bonding if an atom donates an electron it becomes a positively b negatively c stable d neutral 50 Which element is most likely to form a covalent bond hydrogen charged

Inorganic Chemistry
S Block - Group 1All molecules in a pure substance will have the same kinetic energy O True O False

Inorganic Chemistry
S Block - Group 1Polar liquids and nonpolar liquids repel each other O True O False

Inorganic Chemistry
S Block - Group 12 How many sulfur atoms are in 3 00 g of iron pyrite FeS2 M 120 0 A 7 53 1021 C 3 01 x 10 2 B 1 51 x 1022 D 6 02 x 1023

Inorganic Chemistry
S Block - Group 14 Why does the developing chamber in the chromatography procedure need to be kept covered

Inorganic Chemistry
S Block - Group 1Which of these gases will effuse most quickly Br O Xe O 02 O H O
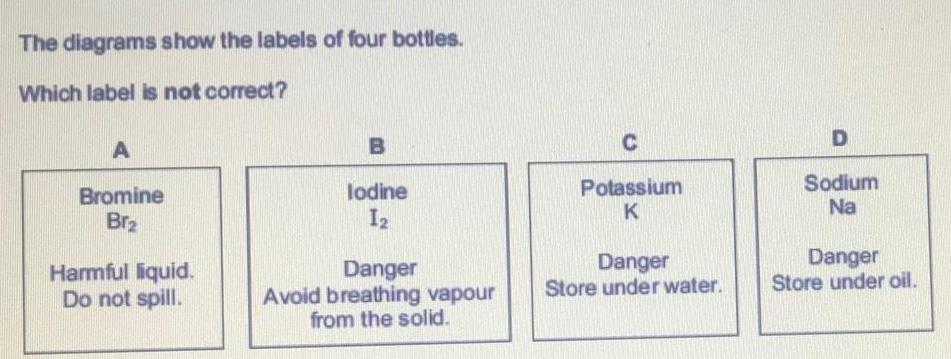
Inorganic Chemistry
S Block - Group 1The diagrams show the labels of four bottles Which label is not correct A Bromine Brz Harmful liquid Do not spill B lodine 12 Danger Avoid breathing vapour from the solid C Potassium K Danger Store under water D Sodium Na Danger Store under oil

Inorganic Chemistry
S Block - Group 1Calculate the K for lactic acid HC3H5Os if in a 1 0000 M solution there is 0 9884 M HC H Os left at equilibrium HC H5O3 H O H 0 C H O 55 5 M start equilibrium A 7 35 x 103 B 1 17 x 102 C 1 36 x 104 10 8 1 0000 M 0 9884 M 55 5 M 0 0 0116 M 0 0 0116 M

Inorganic Chemistry
S Block - Group 1Hunter and Tyler are brothers in their thirties. They had a falling out last year because Tyler felt that
Hunter was rude to his wife. They no longer speak to one another as a result of the conflict. What
type of adult sibling relationship do Hunter and Tyler have?
hostile
competitive
supportive
longing
apathetic

Inorganic Chemistry
S Block - Group 1Copper(II) phthalocyanine (Cu(C32H16N3)) is produced by the cyclotetramerization of phthalonitrile (CsH4N2) according to the following reaction:
C8H4N2(l) + CuCl2(s)→ Cu(C32H16N8)(s) + Cl2(g)
How many grams of copper(II) phthalocyanine would be produced by the complete cyclotetramerization of 6.790 moles of phthalonitrile in the presence of excess copper(II) chloride?

Inorganic Chemistry
S Block - Group 1Which of the following set of quantum numbers (ordered n, l, me, m.) are possible for an electron in an atom?
Check all that apply.
5, 3, -3, 1/2
2, 1, 0, -1
3, 2, 2, -1/2
2, 2, 2, 1/2
4, 2, 3, -1/2
3, 1, -2, -1/2
-1, 0, 0, -1/2
5, 3, 0, 1/2

Inorganic Chemistry
S Block - Group 1An electron resides in a 2p orbital. Which of the following statements is true?
It has a charge equal to a proton, p
The electron is in subshell = 2
The electron is in an orbital with a principal quantum number = 2
The electron is in the lowest energy subshell.
The electron is in the highest energy subshell.

Inorganic Chemistry
S Block - Group 1Consider the following reaction:
2 KCIO3(s) -> 2 KCI (s) + 3 O2 (g)
For this reaction, the oxygen gas produced is collected over water at 27.0 °C in a 3.44 L vessel at a
total pressure of 750.0 torr. How many grams of KCIO3 were consumed in the reaction? The vapor
pressure of water at 27.0 °C is 26.0 torr.
R = 0.08206 L atm/mol K
Do NOT include units in your answer. If you round during your calculations make sure to keep at least
3 decimal places. Report your answer to one (1) decimal place.

Inorganic Chemistry
S Block - Group 1A chemistry student weighs out 0.0825 g of hypobromous acid (HBrO) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1500 M NaOH solution.
Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits.

Inorganic Chemistry
S Block - Group 1A chemist makes 430. mL of silver nitrate (AgNO3) working solution by adding distilled water to 170. mL of a 5.01 M stock solution of silver nitrate in water.
Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.

Inorganic Chemistry
S Block - Group 1In a car engine, gasoline (represented by
C8H18) does not burn completely, and some
CO, a toxic pollutant, forms along with
CO₂ and
H2₂O. If
5.0% of the gasoline forms
CO:
(a) What is the ratio of
CO₂ to
CO molecules in the exhaust?
(b) What is the mass ratio of
CO₂ to
CO?
(c) What percentage of the gasoline must form
CO for the mass ratio of
CO₂ to
CO to be exactly 1/1?

Inorganic Chemistry
S Block - Group 1If the temperature of a bowl of ice cream increases from -10°C to 25°C, what is the increase in temperature in units of degrees Celsius and Kelvin?
15°C, 288 K
35°C, 308 K
35°C, 273 K
35°C, 35 K
15°C, 273 K

Inorganic Chemistry
S Block - Group 1A line in the spectrum of atomic mercury has a wavelength of 254 nm. When mercury emits a photon of light at this wavelength, the frequency of this light is:
1.18 x 10^15 s^-1
7.83 x 10^-19 s^-¹
8.47 x 10^-16 s^-1
76.1 s^-1
None of these choices are correct.

Inorganic Chemistry
S Block - Group 1For the elements Cs, F, and S, the order of increasing electronegativity is:
Cs < F< S
OS< F< Cs
CS <S<F
F<Cs < S
None of these choices are correct.

Inorganic Chemistry
S Block - Group 1For each of the following compounds, classify it with the atomic-scale picture that best represents it in solution or as insoluble in aqueous solution.

Inorganic Chemistry
S Block - Group 1Which of the following properties of a real gas is related to the b coefficient in the van der Waals equation?
The average speed of the molecules of a real gas increases with temperature.
Real gases consist of molecules or atoms that have volume.
There are attractive forces between atoms or molecules of a real gas.
The rate of effusion of a gas is inversely proportional to the square root of the molecular weight of the gas.
None of these choices are correct.

Inorganic Chemistry
S Block - Group 1The term urbanization refers to:
a. farming becoming obsolete.
b. the forced movement of farmers from rural areas to cities.
c. the expansion of cities.
d. a loosening of traditional values in rural communites.

Inorganic Chemistry
S Block - Group 1Which of the following describes the element I?
a. consists of diatomic molecules in elemental form
b. reacts vigorously with alkali metals to form salts
c. forms a basic solution in water
d. is one of the group of the least reactive elements
e. belongs to a group consisting entirely of gases
f. is very reactive as a metal

Inorganic Chemistry
S Block - Group 1Read the following paragraph from the article.
Collier said that in the legends of
the expedition, York's role has been
overlooked. The bust "is really
furthering that conversation here in
our very, very white city."
Which statement is an accurate explanation of
what this paragraph means?
A The York bust is bringing more attention to
the role he played in the expedition.
B The York bust is making people realize that
York was the real leader of the expedition.
C Most people in Portland are not interested in
learning about York's role in the expedition.
D Most people in Portland are not happy
about the York bust appearing in their city.

Inorganic Chemistry
S Block - Group 1Choose the option that best completes the sentence.
The author lists all of the following as reasons to play games, except_
A) learning or practicing a new skill
B) interacting with others
C) getting out of the house
D) having fun

Inorganic Chemistry
S Block - Group 1List and describe your favorite lift from this lesson or your own personal experience. 2 Search Google (or other search engine) and search "body weight exercises" and try them out. Describe your experience with at least one of them. 3 Discuss how you will (or are already) using body weight exercises in your life.

Inorganic Chemistry
S Block - Group 1Choose the option that best completes each sentence.
The author states all of the following except
A) the history of violence and taking risks teaches us how to prolong our ives
B) artists often talk about previous artists' influence on their work
C) we cannot learn from the history of governments and politics
D) we can learn how to preserve our environment by learning from previous
mistakes

Inorganic Chemistry
S Block - Group 1A student performs the experiment and records the following data:
Barometric pressure: 741.6 torr
Temperature of water bath: 99 °C
Volume of flask: 273 mL
Mass of condensed vapor: 0.65 g
a. The student calculates the molecular weight of the gas to be 2.6 x 10-5 g/mol.
What mistake did he make?
![M (Alkali metal) +(X+Y) NH3 → [M(NH3)x]* +[e(NH3)y]
Mark the incorrect statement about the solution
solution has high electric conductivity due to ammoniated electrons
dilute solution imparts copper Bronze colour
Dilute solution is paramagnetic
On the addition of substances like 'Iron' the solution decomposes and releases H₂ gas](https://media.kunduz.com/media/sug-question/raw/54571286-1657520207.6881943.jpeg?w=256)
Inorganic Chemistry
S Block - Group 1M (Alkali metal) +(X+Y) NH3 → [M(NH3)x]* +[e(NH3)y]
Mark the incorrect statement about the solution
solution has high electric conductivity due to ammoniated electrons
dilute solution imparts copper Bronze colour
Dilute solution is paramagnetic
On the addition of substances like 'Iron' the solution decomposes and releases H₂ gas
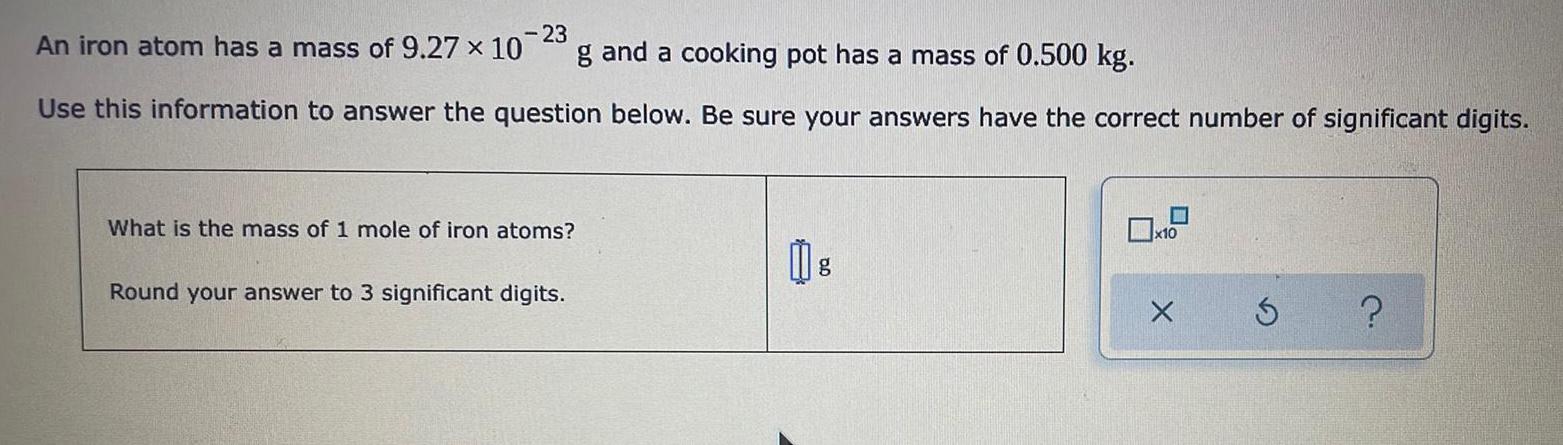
Inorganic Chemistry
S Block - Group 1An iron atom has a mass of 9.27 x 10^-23 g and a cooking pot has a mass of 0.500 kg.
Use this information to answer the question below. Be sure your answers have the correct number of significant digits.

Inorganic Chemistry
S Block - Group 1Arrange the following bonds from the least polar to the most polar bond: N-F, Na-F, As-F, P-F, F-F
WHERE ARE WE GOING?
What information do we need to arrange the bonds from the least polar to the most polar bond?
(Select all that apply.)
the given bonds in the problem
the atomic size of each element
the Pauling electronegativity values
the molar mass of each element
the number of electrons for each element

Inorganic Chemistry
S Block - Group 12- The following data set are four replicas for the measurements of the concentration in Molarity of NaCl in Human Serum (0.163, 0.164, 0.166, and 0.169) calculate:
a) The arithmetic mean or sample mean
b) The Median
c) The sample standard deviation
d) The range or spread
e) Conduct the t-student's test with 95% confidence

Inorganic Chemistry
S Block - Group 1Which of the following is incorrect statement about lithium?
Melting and boiling point of lithium is higher than other alkali metals
LiCl is deliquescent and crystallises as a hydrate LiCI.2H2O
Lithium hydrogen carbonate is thermally most stable among alkali metal hydrogen carbonate
Lithium nitrate on heating produces Li2O, NO2 and O2

Inorganic Chemistry
S Block - Group 1Why does KBr have a lower melting point temperature than NaCl?
a. Because the electron transfer, a requirement for ionic bonding, is less complete for KBr salts
b. Because KBr salts ions with greater charge than those found in NaCl.
c. Because period 4 elements have lower melting point temperatures as a general rule
d. Because KBr salts are composed of larger ions than NaCl salts.
e. Because NaCl salts utilize expanded ionic bonding; something not possible for KBr salts

Inorganic Chemistry
S Block - Group 1What is the electron geometry of CIF5 ?
What is the molecular geometry of CIF5?
Ignoring lone-pair effects, what is the smallest bond angle in CIF5 ?

Inorganic Chemistry
S Block - Group 1The first ionization energy of lithium is low. The second ionization energy of lithium is high. Based on this pattern, which of the following is the most likely behavior for lithium?
A. Lose one electron.
B. Gain one electron.
C. Lose two electrons.
D. Gain two electrons.

Inorganic Chemistry
S Block - Group 1Naturally occurring oxygen consists primarily of three isotopes of mass numbers 16, 17, and 18. How many protons does each of these nuclides contain? How many neutrons does each of these nuclides contain? Choose nuclear symbols for each of these isotopes.
(Select all that apply.)
A. 8O17 (8 protons, 9 neutrons)
B. 8O18 (8 protons, 10 neutrons)
C. 8O16 (8 protons, 8 neutrons)
D. 8O17 (8 protons, 16 neutrons)
E. 8O18 (8 protons, 18 neutrons)
1