Math - Others Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Math - Others
Basic MathK ces Exhibit 4 13 Work Breakdown Structure and Activities for the Cell Phone Design Project ACTIVITY IDENTIFICATION MAJOR SUBPROJECTS ACTIVITIES Product Specifications P Product design D Supplier specifications S Market research Overall product specifications Hardware Software Subcontracting V Product integration I Hardware Software Battery Display Camera Outer cover Hardware User interface Software coding Prototype testing Minimum number of weeks Suppliers selection Contract negotiation P1 P2 P3 P4 S1 S2 D1 D2 D3 D4 I1 12 13 14 V1 V2 a Determine the minimum number of weeks for completing the project DEPENDENCY 1 P1 P2 P3 P4 P4 S1 S1 S1 D1 D2 D3 D4 D2 12 11 13 S1 S2 14 V1 DURATION WEEKS 3 5 5 5 5 7 3 5 W5 SSSN S 2 5 5 5 10

Math - Others
Basic Math20 How many moles of carbon tetrachloride CCL is represented by 543 2 g of carbon tetrachloride The atomic weight of carbon is 12 01 and the atomic weight of chlorine is 35 45 OA 5 42 moles B 3 53 moles C 8 35x104 moles OD 11 4 moles

Math - Others
Basic Math16 The United States National Weather Service reports pressure in mm of Hg What s the pressure of 725 mmHg in Pa millibars and Torr OA 96 657 Pa 966 millibar 725 Torr OB 100 000 Pa 1000 millibar 760 Torr C 100 000 Pa 760 millibar 725 Torr D 101 325 Pa 966 millibar 725 Torr

Math - Others
Basic Math10 What property of gases confirms that the space between the particles of gas is significantly larger than when the same substance is in a liquid state O A Mobility O B Volatility O C Compressibility D Reactivity

Math - Others
Basic Math12 A flask containing 100 g of water is heated and the temperature of the water increases from 21 C to 90 C How much heat did the water absorb if the specific heat capacity of water is 4 184 J g C A 28 870 J O B 289 J O C 10 000 J O D 418 4 J

Math - Others
Basic Math3 What s the mass in grams of 0 442 moles of calcium bromide CaBr The atomic weight of Ca is 40 1 and the atomic weight of Br is 79 9 OA 53 04 g OB 88 4 g C 44 2 g O D 452 3 g

Math - Others
Basic Math7 What s the temperature in degrees Celsius of an 11 2 L sample of CO at 744 torr if it occupies 13 3 L at 55 C and 744 torr O A 3 C B 10 C O C 0 C O D 5 C

Math - Others
Basic Math19 Stoichiometry is the study of chemical reactions that OA lists OB multiplies OC divides OD counts the particles

Math - Others
Inverse Trigonometric functions5 At a fixed volume a four fold increase in the temperature of a gas will lead to OA a two fold decrease OB a four fold decrease OC a four fold increase O D no change in pressure

Math - Others
Basic MathSelect the best answer for the question 9 A gas in a 6 2 mL cylinder has a pressure of 1 4 atmospheres A piston is pushed in until the gas volume is 3 1 mL while the temperature remains constant What s the final pressure of the gas in the cylinder OA 5 6 atm OB 2 8 atm OC 4 8 atm OD 3 2 atm

Math - Others
Binomial theoremL atm 17 A 0 223 mole sample of a gas is held at 33 0 C and 2 00 atm what s the volume of the gas R 0 0821 mol K A 5 60 L B 2 80 L C 0 302 L D 0 604 L

Math - Others
Basic Math11 One day in the chemistry lab students put a few pieces of dry ice into a beaker Dry ice is solid frozen carbon dioxide They see vapor rising from the dry ice but no liquid as it slowly disappears into the air at room temperature What kind of phase transition did the students observe O A Melting B Evaporation C Deposition COD Sublimation

Math - Others
Basic Math18 One mole of copper has a different mass from one mole of mercury because O A copper has a greater affinity for mercury and therefore will have a different mass OB the mass is based upon the chemical interactions of copper and mercury OC moles are different numbers depending on the atom OD an atom of mercury has a different mass than an atom of copper

Math - Others
Basic Math2 What s the empirical formula of a compound composed of 22 55 P and 77 45 CI O A PC1 OB PCI O C PCI O D PCI5

Math - Others
Basic Math14 Stoichiometry is based on O A pressure OB temperature 0 C molecular weight D conservation of matter

Math - Others
Basic MathVolume A O A Graph B O B Graph C Volume C Graph A D Graph D B Volume Number of moles Number of moles Number of moles Number of moles 13 Which graph shows the volume amount of gas relationship expected for an ideal gas also known as Avogadro s law Volume

Math - Others
Basic Math1 A sample of wax requires heat to melt and become a liquid What type of process is this OA Exothermic OB Sublimation OC Deposition OD Endothermic

Math - Others
Basic Math6 Consider a cup of hot tea a cup of juice at room temperature and a cup of ice water Which beverage has the highest average kinetic energy O A All liquids have the same average kinetic energy OB A cup of ice water OC A cup of hot tea O D A cup of juice at room temperature

Math - Others
Basic MathB At what geographical location would the boiling point of water be lowest A The coast of the Atlantic Ocean OB The Dead Sea OC The top of Mount Everest OD Boston Massachusetts

Math - Others
Inverse Trigonometric functionsGraph the following inequality Then write the inequality in interval notation 8 x27 Choose the correct graph below OA 1 OC 1 2 2 3 3 4 4 5 6 5 6 The inequality can be written as Type your answer in interval notation 7 7 8 8 9 9 OB 10 10 1 OD 1 2 2 3 4 5 3 4 5 6 6 7 8 8 9 9

Math - Others
Basic MathWrite the following inequality in interval notation Then graph the inequality 6 x 8 The inequality can be written as Type your answer in interval notation Choose the correct graph below OA 1 OC 1 2 2 3 3 4 4 5 45 6 96 7 7 778 8 100 8 9 10 9 10 OB 1 OD 1 2 2 3 3 4 4 5 ti 5 6 916 7 718 30 7 8 S

Math - Others
Basic MathWrite the inequality in interval notation Graph the inequality x 1 The solution set is Choose the correct graph below O A O C 10 8 6 4 2 4 10 Type your answer in interval notation 8 4 6 1 0 2 4 6 8 2 0 2 4 6 8 A 10 10 OB O D 10 10 8 8 4 6 6 4 2 2 1 T 1 0 0 2 12 st 4 6 4 6

Math - Others
Basic Math7 In KCI how are the valence electrons distributed O A The electrons are equally shared between K and Cl forming a covalent bond OB The electrons are transferred from K to Cl OC The electrons are unequally shared between K and Cl forming a polar covalent bond O D The electrons are shared between many K and Cl ions creating a sea of electrons

Math - Others
Mathematical Induction18 Which portion of a molecule of F O has partial positive charge OA The central O atom OB The partial charge on each atom is negative C The F atoms O D The partial charge on each atom is zero

Math - Others
Basic Math10 What s the structure of CI O Is it polar or non polar O A Bent or angular non polar B Bent or angular polar OC Trigonal planar polar O D Trigonal planar non polar

Math - Others
Basic Math6 In the reaction Cal s Cl g CaCl s 1 s what phases are the products in after the reaction O A Both products are liquids O B Both products are solids C Cal is a solid and CaCl is in aqueous solution O D CaCl is a liquid and l is a gas

Math - Others
Functions19 A chemical reaction has the equation 4AI s 3 O g 2Al O s What type of reaction is this O A Double displacement O B Synthesis OC Single displacement D Decomposition

Math - Others
Mathematical Induction2 For an ion the sum of the formal charges on each atom should equal O A the overall charge of the ion O B the electronegativity of each atom in the ion OC the opposite of the overall charge of the ion D zero

Math - Others
Functions16 Two atoms bonded together will remain some distance apart minimizing the O A potential energy of the bond OB number of valence electrons in the bond OC bond distance O D partial charge of the bond

Math - Others
Basic Math17 Which of the following chemical reactions is a single displacement reaction O A 2Al 3Zn NO3 2 2AI NO3 3 3Zn OB 2Fe OH 3 Fe O3 3H O O C Pb NO3 2 2KBr PbBr 2KNO3 O D 2Na O Na O

Math - Others
Basic Math15 Which type of bonding would occur between multiple copper atoms O A Covalent OB Metallic C Ionic D Polar covalent

Math - Others
Basic Math9 A reversible reaction has a forward rate constant of 0 412 mol L s and a reverse reaction rate constant of 0 827 mol L s What s the equilibrium constant for this reaction O A 1 417 O B 2 007 C 0 498 O D 0 706

Math - Others
Basic Math4 What s the structure of PF Is it polar or non polar O A Trigonal bipyramidal non polar OB Square pyramid polar O C Trigonal bipyramidal polar OD Square pyramid non polar

Math - Others
Basic Math14 Which process is used to produce gases from solutions of salts dissolved in water or another liquid O A lonic bonding O B Polar covalent bonding O C Metallic bonding O D Electrolysis

Math - Others
Binomial theorem13 In a certain chemical reaction 2 hydrogen chloride molecules in aqueous solution react with solid zinc The reaction produces zinc chloride in aqueous solution and hydrogen gas Which of the following reaction equations correctly describes this reaction O A 2HCI aq Zn s ZnCl aq H g O B HCI aq Zn s Zn Cl aq 2H 1 O C HCI aq 2Zn s 2ZnCl aq H g O D 2HCl g 2Zn s 2ZnCl s H g

Math - Others
Mathematical Induction3 Set up for the PCR tubes for a Obtain 1 PCR tube containing Ready To Go PCR beads for each sample three in total and label with the sample type group color and tubulin b Add 22 5 uL of the tubulin primer loading dye mix to each tube Allow several min for bead to dissolve c Add 2 5 L of each DNA sample to its respective tube and label 4 Close each tube and mix by tapping the bottom of the tube Keep tubes on ice until all groups are ready for the next step 5 Give your tubes to your TA to place into the Thermocycler The amplification run setup is as follows PCR Cycle Program 1 cycle 95 C 5 min 36 cycles 94 C 10 sec 55 C 5 sec 72 C 5 sec 1 cycle 72 C 1 min The PCR cycle includes a denaturation step 94 C a reannealing step 55 C and an elongation step 72 C PCR Beads contain PuReTaq DNA Polymerase 10 mM Tris HCl 50 mM KCl 1 5 mM MgCl2 200 M of each dNTP deoxyribunucleated triphosphorylated DNA bases Preparing for Gel Electrophoresis Make the following solution 75 ml 10x TBE Tris base Boric acid EDTA 0 9 M Tris 0 889 M boric acid 20 mM EDTA This solution will be used next week for the gel electrophoresis in Part C of the protocol

Math - Others
Basic MathMAT276 Modern Differential Equations Modeling Scenario 1 Consider a regular size bag of M M candies not peanut just regular Usually there are about 55 pieces in each bag They are of different colors but each piece almost always has an m pressed on one side and not the other Hence there are distinctions between the sides Let us conduct a couple of experiments life and death experiments on the M Ms 1 Model 1 Death Model sounds ominous right Put 50 M M s into a small container like a paper cup First just read the description of the experiment Before actually doing anything describe what you think will happen and offer up some assumptions in support of your description some which might not have that much to do with what you expect to happen Description of the Experiment don t do it yet Gently shake the M Ms out onto a flat surface like a desk or you might want to use a paper plate to catch the M Ms and keep them clean as well We determine for each M M if it lives or dies If the m shows on top this M M dies otherwise there is life for this M M Upon death you should remove the M M from the population set these aside as we will need them for another experiment count and note down the number of M Ms who survive in Table 1 and thus put fewer M Ms back into your container for the next iteration Do this over and over and record your results Questions 1 State your assumptions about the physical activity Many times assumptions run into each other so break the assumptions down to their simplest form i e do not use words like and 2 Offer up a description in words of what should happen Care to make a prediction about what happens in the long run Adapted from 1 1 M M DeathImmigration Parameter Estimation SIMIODE modeling scenario by Dr Brian Winkel Professor Emeritus USMA Director SIMIODE 1

Math - Others
Basic Math3 7 12 Find the lowest common denominator of and 3 x y O A x y5 O B xy4 O C x y4 O D x y

Math - Others
Basic Math15 Change the fraction O A B C D w 7w 12 w w 20 w 7w 12 w w 20 w 7w 12 w w 20 w 7w 12 w w 20 w 3 w 5 into an equivalent fraction with the denominator w w 20

Math - Others
Basic Math9 Find the illegal values of b in the fraction OA b 2 and 4 OB B 5 2 2 and 4 OC b 5 and 2 OD b 2 and 4 26 36 10 6 26 8

Math - Others
Basic Math19 Simplify the following expression 0 A B C D 26 9 11 144 75 131 3 14 2 5 35 43

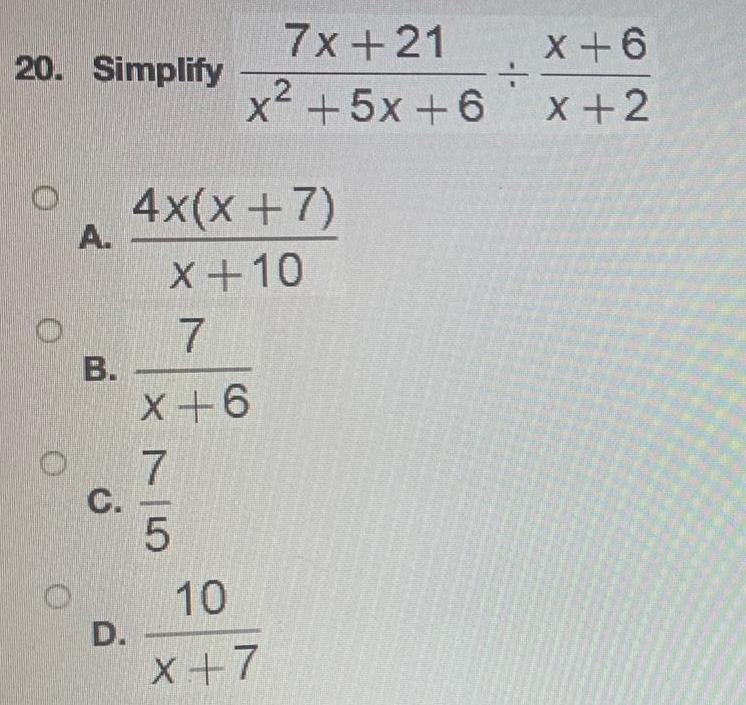

Math - Others
Basic Math10 Find the illegal values of c in the multiplication statement O A c 7 c 3 O B c 7 c 3 c 2 and c 5 O C c 7 c 3 c 2 and c 5 OD c 7 c 3 c 2 and c 5 c 3c 10 c c 2 c 5c 14 c 2c 15


Math - Others
Basic Math11 Divide O O O A B C D r 5 2 5r 14 r 5 r 3 r 5 r 3 r 2 by r 5 r 7 r 7 r 3 r 5 r 7 r 3 r 4r 21 r 2


Math - Others
Basic Math4 13 Simplify the following expression 1 O 0 A B C D 16 9 189 400 100 601 4 3 52 4 2

