Basic Math Questions and Answers

Math - Others
Basic MathK ces Exhibit 4 13 Work Breakdown Structure and Activities for the Cell Phone Design Project ACTIVITY IDENTIFICATION MAJOR SUBPROJECTS ACTIVITIES Product Specifications P Product design D Supplier specifications S Market research Overall product specifications Hardware Software Subcontracting V Product integration I Hardware Software Battery Display Camera Outer cover Hardware User interface Software coding Prototype testing Minimum number of weeks Suppliers selection Contract negotiation P1 P2 P3 P4 S1 S2 D1 D2 D3 D4 I1 12 13 14 V1 V2 a Determine the minimum number of weeks for completing the project DEPENDENCY 1 P1 P2 P3 P4 P4 S1 S1 S1 D1 D2 D3 D4 D2 12 11 13 S1 S2 14 V1 DURATION WEEKS 3 5 5 5 5 7 3 5 W5 SSSN S 2 5 5 5 10

Math - Others
Basic Math20 How many moles of carbon tetrachloride CCL is represented by 543 2 g of carbon tetrachloride The atomic weight of carbon is 12 01 and the atomic weight of chlorine is 35 45 OA 5 42 moles B 3 53 moles C 8 35x104 moles OD 11 4 moles

Math - Others
Basic Math16 The United States National Weather Service reports pressure in mm of Hg What s the pressure of 725 mmHg in Pa millibars and Torr OA 96 657 Pa 966 millibar 725 Torr OB 100 000 Pa 1000 millibar 760 Torr C 100 000 Pa 760 millibar 725 Torr D 101 325 Pa 966 millibar 725 Torr

Math - Others
Basic Math10 What property of gases confirms that the space between the particles of gas is significantly larger than when the same substance is in a liquid state O A Mobility O B Volatility O C Compressibility D Reactivity

Math - Others
Basic Math12 A flask containing 100 g of water is heated and the temperature of the water increases from 21 C to 90 C How much heat did the water absorb if the specific heat capacity of water is 4 184 J g C A 28 870 J O B 289 J O C 10 000 J O D 418 4 J

Math - Others
Basic Math3 What s the mass in grams of 0 442 moles of calcium bromide CaBr The atomic weight of Ca is 40 1 and the atomic weight of Br is 79 9 OA 53 04 g OB 88 4 g C 44 2 g O D 452 3 g

Math - Others
Basic Math7 What s the temperature in degrees Celsius of an 11 2 L sample of CO at 744 torr if it occupies 13 3 L at 55 C and 744 torr O A 3 C B 10 C O C 0 C O D 5 C

Math - Others
Basic Math19 Stoichiometry is the study of chemical reactions that OA lists OB multiplies OC divides OD counts the particles

Math - Others
Basic MathSelect the best answer for the question 9 A gas in a 6 2 mL cylinder has a pressure of 1 4 atmospheres A piston is pushed in until the gas volume is 3 1 mL while the temperature remains constant What s the final pressure of the gas in the cylinder OA 5 6 atm OB 2 8 atm OC 4 8 atm OD 3 2 atm

Math - Others
Basic Math11 One day in the chemistry lab students put a few pieces of dry ice into a beaker Dry ice is solid frozen carbon dioxide They see vapor rising from the dry ice but no liquid as it slowly disappears into the air at room temperature What kind of phase transition did the students observe O A Melting B Evaporation C Deposition COD Sublimation

Math - Others
Basic Math18 One mole of copper has a different mass from one mole of mercury because O A copper has a greater affinity for mercury and therefore will have a different mass OB the mass is based upon the chemical interactions of copper and mercury OC moles are different numbers depending on the atom OD an atom of mercury has a different mass than an atom of copper

Math - Others
Basic Math2 What s the empirical formula of a compound composed of 22 55 P and 77 45 CI O A PC1 OB PCI O C PCI O D PCI5

Math - Others
Basic Math14 Stoichiometry is based on O A pressure OB temperature 0 C molecular weight D conservation of matter

Math - Others
Basic MathVolume A O A Graph B O B Graph C Volume C Graph A D Graph D B Volume Number of moles Number of moles Number of moles Number of moles 13 Which graph shows the volume amount of gas relationship expected for an ideal gas also known as Avogadro s law Volume

Math - Others
Basic Math1 A sample of wax requires heat to melt and become a liquid What type of process is this OA Exothermic OB Sublimation OC Deposition OD Endothermic

Math - Others
Basic Math6 Consider a cup of hot tea a cup of juice at room temperature and a cup of ice water Which beverage has the highest average kinetic energy O A All liquids have the same average kinetic energy OB A cup of ice water OC A cup of hot tea O D A cup of juice at room temperature

Math - Others
Basic MathB At what geographical location would the boiling point of water be lowest A The coast of the Atlantic Ocean OB The Dead Sea OC The top of Mount Everest OD Boston Massachusetts

Math - Others
Basic MathWrite the following inequality in interval notation Then graph the inequality 6 x 8 The inequality can be written as Type your answer in interval notation Choose the correct graph below OA 1 OC 1 2 2 3 3 4 4 5 45 6 96 7 7 778 8 100 8 9 10 9 10 OB 1 OD 1 2 2 3 3 4 4 5 ti 5 6 916 7 718 30 7 8 S

Math - Others
Basic MathWrite the inequality in interval notation Graph the inequality x 1 The solution set is Choose the correct graph below O A O C 10 8 6 4 2 4 10 Type your answer in interval notation 8 4 6 1 0 2 4 6 8 2 0 2 4 6 8 A 10 10 OB O D 10 10 8 8 4 6 6 4 2 2 1 T 1 0 0 2 12 st 4 6 4 6

Math - Others
Basic Math7 In KCI how are the valence electrons distributed O A The electrons are equally shared between K and Cl forming a covalent bond OB The electrons are transferred from K to Cl OC The electrons are unequally shared between K and Cl forming a polar covalent bond O D The electrons are shared between many K and Cl ions creating a sea of electrons

Math - Others
Basic Math10 What s the structure of CI O Is it polar or non polar O A Bent or angular non polar B Bent or angular polar OC Trigonal planar polar O D Trigonal planar non polar

Math - Others
Basic Math6 In the reaction Cal s Cl g CaCl s 1 s what phases are the products in after the reaction O A Both products are liquids O B Both products are solids C Cal is a solid and CaCl is in aqueous solution O D CaCl is a liquid and l is a gas

Math - Others
Basic Math17 Which of the following chemical reactions is a single displacement reaction O A 2Al 3Zn NO3 2 2AI NO3 3 3Zn OB 2Fe OH 3 Fe O3 3H O O C Pb NO3 2 2KBr PbBr 2KNO3 O D 2Na O Na O

Math - Others
Basic Math15 Which type of bonding would occur between multiple copper atoms O A Covalent OB Metallic C Ionic D Polar covalent

Math - Others
Basic Math9 A reversible reaction has a forward rate constant of 0 412 mol L s and a reverse reaction rate constant of 0 827 mol L s What s the equilibrium constant for this reaction O A 1 417 O B 2 007 C 0 498 O D 0 706

Math - Others
Basic Math4 What s the structure of PF Is it polar or non polar O A Trigonal bipyramidal non polar OB Square pyramid polar O C Trigonal bipyramidal polar OD Square pyramid non polar

Math - Others
Basic Math14 Which process is used to produce gases from solutions of salts dissolved in water or another liquid O A lonic bonding O B Polar covalent bonding O C Metallic bonding O D Electrolysis

Math - Others
Basic MathMAT276 Modern Differential Equations Modeling Scenario 1 Consider a regular size bag of M M candies not peanut just regular Usually there are about 55 pieces in each bag They are of different colors but each piece almost always has an m pressed on one side and not the other Hence there are distinctions between the sides Let us conduct a couple of experiments life and death experiments on the M Ms 1 Model 1 Death Model sounds ominous right Put 50 M M s into a small container like a paper cup First just read the description of the experiment Before actually doing anything describe what you think will happen and offer up some assumptions in support of your description some which might not have that much to do with what you expect to happen Description of the Experiment don t do it yet Gently shake the M Ms out onto a flat surface like a desk or you might want to use a paper plate to catch the M Ms and keep them clean as well We determine for each M M if it lives or dies If the m shows on top this M M dies otherwise there is life for this M M Upon death you should remove the M M from the population set these aside as we will need them for another experiment count and note down the number of M Ms who survive in Table 1 and thus put fewer M Ms back into your container for the next iteration Do this over and over and record your results Questions 1 State your assumptions about the physical activity Many times assumptions run into each other so break the assumptions down to their simplest form i e do not use words like and 2 Offer up a description in words of what should happen Care to make a prediction about what happens in the long run Adapted from 1 1 M M DeathImmigration Parameter Estimation SIMIODE modeling scenario by Dr Brian Winkel Professor Emeritus USMA Director SIMIODE 1

Math - Others
Basic Math3 7 12 Find the lowest common denominator of and 3 x y O A x y5 O B xy4 O C x y4 O D x y

Math - Others
Basic Math15 Change the fraction O A B C D w 7w 12 w w 20 w 7w 12 w w 20 w 7w 12 w w 20 w 7w 12 w w 20 w 3 w 5 into an equivalent fraction with the denominator w w 20

Math - Others
Basic Math9 Find the illegal values of b in the fraction OA b 2 and 4 OB B 5 2 2 and 4 OC b 5 and 2 OD b 2 and 4 26 36 10 6 26 8

Math - Others
Basic Math19 Simplify the following expression 0 A B C D 26 9 11 144 75 131 3 14 2 5 35 43

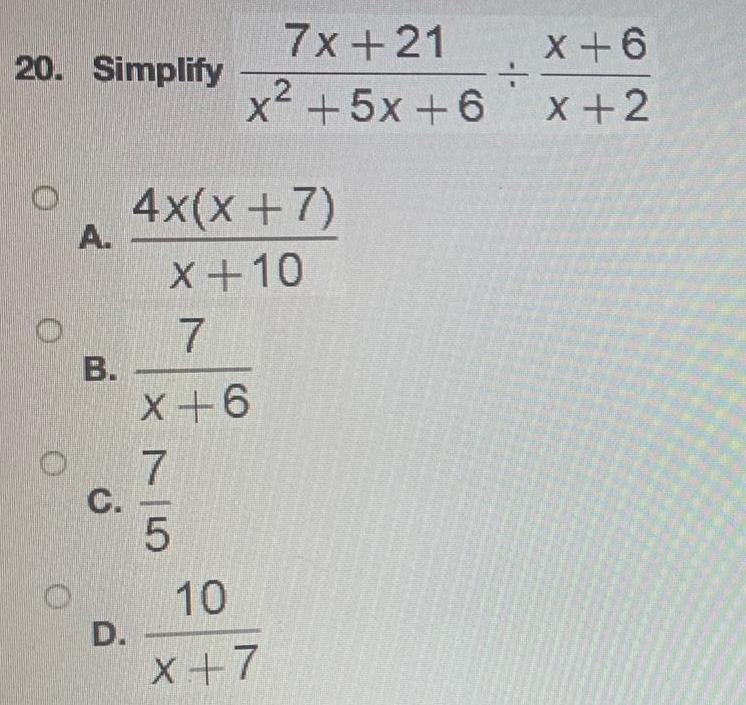

Math - Others
Basic Math10 Find the illegal values of c in the multiplication statement O A c 7 c 3 O B c 7 c 3 c 2 and c 5 O C c 7 c 3 c 2 and c 5 OD c 7 c 3 c 2 and c 5 c 3c 10 c c 2 c 5c 14 c 2c 15


Math - Others
Basic Math11 Divide O O O A B C D r 5 2 5r 14 r 5 r 3 r 5 r 3 r 2 by r 5 r 7 r 7 r 3 r 5 r 7 r 3 r 4r 21 r 2


Math - Others
Basic Math4 13 Simplify the following expression 1 O 0 A B C D 16 9 189 400 100 601 4 3 52 4 2



Math - Others
Basic Math4 Simplify A B C D 3 2x 5 15x 5 x 5 2x 5 10x 12 6 x 6 ST X 5 15 x 5 2x 5 13x 10 x 5 2x 5 5



Math - Others
Basic Math3 Multiply Ar 2 C D r 7r 10 by 3 7r 20r 300 5r 5 3r 6 2 r 5 r 2 r 10 3r 30 7 5r 50

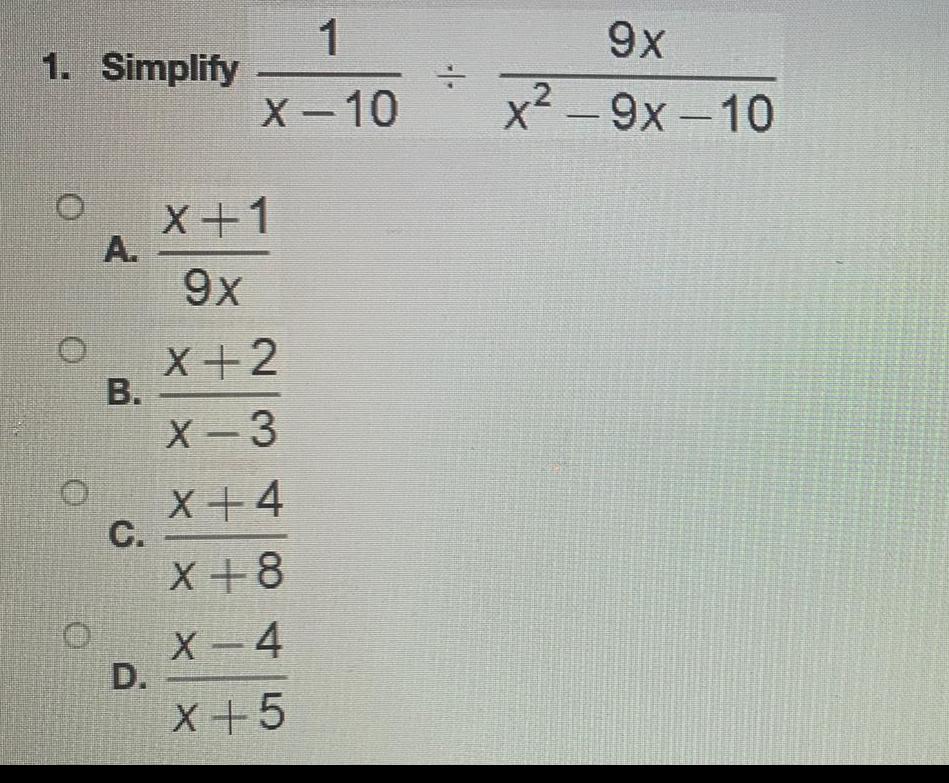


Math - Others
Basic MathA Contractor purchases gravel one cubic yard at a time a A gravel driveway L yards long and 6 yards wide is to be poured to a depth of 1 foot Find a formula for n L the number of cubic yards of gravel th contractor buys assuming that he buys 10 more cubic yards of gravel than are needed to be sure he ll have enough cubic yards help formulas n L b Assuming no driveway is less than 5 yards long or more than 12 yards long find the domain and range of n L Enter your answers as comma separated lists of numbers Domain Range help numbers help numbers c Assuming that no driveway is less than 5 yards long or more than 12 yards long sketch a graph of n L Which graph A D below most closely matches the graph you drew

Math - Others
Basic MathFind the equivalent resultant force for the distributed load acting on the beam O 6 KN O 8 KN O 12 kN 2 kN m A 3 m K 3 m 4 kN m B