Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
BiomoleculesOxyhemoglobin is diamagnetic due to electron spin coupling betwee A Low spin Fe and oxygen molecule B High spin Fe and superoxide radical C High spin Fe2 and oxygen molecule D Low spin Fe and superoxide radical

Organic Chemistry
Hydrocarbons11 Boiling point order of isomeric amine is 1 Primary Secondary Tertiary 2 Primary Secondary Tertiary 3 Primary Secondary Tertiary 4 Primary Secondary Tertiary

Organic Chemistry
General organic chemistryH2N CH2CH2CH CH2 CHO product is Only One Correct Answer B A H N CH CH CH CH2 CH OH C H Ni CN NH A O

Organic Chemistry
Practical DetectionThe standard biological reaction Gibbs energy for the removal of the phosphate group from adenosine monophosphate is 14 kJ mol 1 at 298K What is the value of the thermodynamic standard reaction Gibbs energy

Organic Chemistry
General organic chemistry13 Correct order of basic nature is 1 C H NH C H5NH C H 2N NH3 2 C H NH C H 3N C H NH NH3 3 C H NH C HyN CH NH NHg 4 NHg C H NH C H NH C HJgN

Organic Chemistry
Aldehydes & KetonesThe following compound was made by a mixed Aldol reaction Identify the starting materials other reagents and their order of use Your synthesis should be designed to give this compound as the exclusive product OH O I

Organic Chemistry
General organic chemistrySerratia marcescens is a bacterial species that produces a red pigment when grown at 25 C and remains colorless at 37 C What is t O Mesophilic bacteria are adapting to a lower temperature The environment is expressing a phenotype A change in the genotype The protein being inactivated at high temperatures O Temperature rearranging the gene sequence

Organic Chemistry
General organic chemistryArrange each set of compounds in order of increasing boiling points CM3 Br 1 Bromomethane Bromoform Chloromethane Dibromomethane ii 1 Chloropropane Isopropyl chloride 1 Chlorobutane

Organic Chemistry
Aldehydes & KetonesIncreasing order of equilibrium constants for the formation of a hydrate dddd NH I a IV III II I I II III IV NH II O N III b IV III I II d II III I IV IV OH

Organic Chemistry
General organic chemistryAs it is directly proportional to no Of pi bond than why be nzene is in middle For the reactions 211 31 Select the correct option Question Type Single Correct Type 1 y z x 2 x z y AH x Kcal mol 1 AH y Kcal mol AH z Kcal mol 3 z x y 4 z y x

Organic Chemistry
General organic chemistryThe addition of a catalyst during a chemical reaction alters which of the following quantities Internal energy Enthalpy Activation energy Entropy

Organic Chemistry
AminesConsider the following information about compound P C4H 1N Number of 1 amines structural only X Number of 2 amines structural only Y Number of 3 amines structural only Z The value of X Y Z is

Organic Chemistry
IsomerismThe heat of hydrogenation for 3 methylbutene and 2 pentene are 30 kcal mol and 28 kcal mol respectively The heats of combustion of 2 methylbutane and pentane are 784 kcal mol and 782 kcal mol respectively All the values are given under standard conditions Taking into account that combustion of both alkanes give the same products what is AH in kcal mol for the following reaction under same conditions a 0 Y b 4 c 2 d 2

Organic Chemistry
General organic chemistryiv none of the above b The correct order of acidic strength among the followings II CH3CH OH III C H OH 1 H O i III IV II I iii IV III II I ii IV III I II iv I II IV III IV p chlorophenol
Organic Chemistry
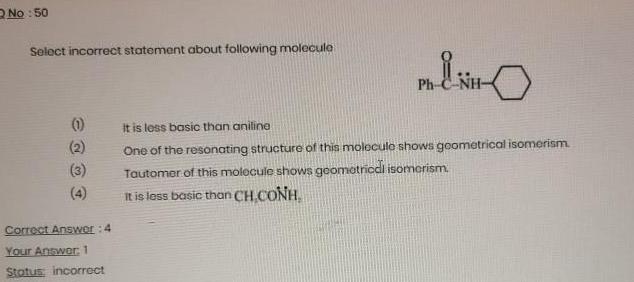
General organic chemistryNo 50 Select incorrect statement about following molecule 1 Correct Answer 4 Your Answer 1 Status incorrect MENO It is less basic than aniline One of the resonating structure of this molecule shows geometrical isomerism Tautomer of this molecule shows geometrical isomerism It is less basic than CH CONH

Organic Chemistry
Chemistry in Daily LifeHydrolysis of ethyl acetate in acidic medium is Fractional order reaction Zero order reaction Pseudo first order reaction hr m Second order reaction

Organic Chemistry
Aldehydes & KetonesAn unsaturated hydrocarbon A absorbs two molecules of hydrogen to give a saturated hydrocarbon and absorbs two molecules of bromine to give a tetrabromo derivative Compound A absorbs two molecules of ozone to give a diozonide The diozonide on warming with Zn H O gives a mixture of three compounds acetaldehyde acetone and propan 1 3 dial What is A

Organic Chemistry
EthersWhich of the following statements about Grignard reagent is not true Select one O a O b Grignard reagents RMgBr add to the carbonyl group of aldehydes and ketones An organosodium compound is not very reactive compared to a Grignard reagent Oc Grignard reagents are decomposed by water and alcohol O d Grignard reagents are prepared in ether or tetrahydrofuran THE

Organic Chemistry
Chemistry in Daily Life58 A Aryl or alkyl monochloride B How many isomers of A will give B When B is a CH3 CH CH3 CH3 c CH3 CH CH CH3 CH3 For example if abcd each are formed from 3 isomers of A in every case fill 3333 i Mg Reflux ii H30 b CH CH Ph PH d Ph CH CH3

Organic Chemistry
Aldehydes & Ketones36 What reagent and or reaction conditions would you choose to bring about the following conversion 0 a 1 LiAlH4 2 H O c H O NaOH heat CH 0 HOCH2CH OH b H O H SO4 heat d PCC CH Cl 2

Organic Chemistry
Hydrocarbons38 Select the chain propagation steps in the free radical chlorination of methane 1 Cl 2C1 2 Cl CH CH Cl H 4 3 Cl CH 4 H Cl HCl Cl 5 CH3 Cl a 2 3 5 e 3 5 CH CH3 HCl CH3Cl Cl b 1 3 6 d 2 3 4

Organic Chemistry
General organic chemistry4 In ethers the grou considered as a substituent in RH where a R is higher than R b R and R are same c R may be higehr than or same as R d R is smaller than R

Organic Chemistry
General organic chemistryWhich is the correct order of increasing basicity A CH3CH CH3 CH3CH SH CH3CH OH CH3CH2NH2 B CH3CH CH3 CH3CH OH CH3CH SH CH3CH NH2 C CH3CH NH2 CH3CH SH CH3CH OH CH3CH CH3 D CH3CH CH3 CH3CH OH CH3CH NH CH3CH SH

Organic Chemistry
Reactions of benzeneE CAPS 40 A Chemistry 7 In which of the following reactions product shown is the major product CHO CH NO COOCH3 OH Cl FeCl Conc H SO4 Conc HNO3 Conc H SO4 Conc HNO3 Conc H SO4 CHO CH3 NO NO CI COOCH OH SO H NO

Organic Chemistry
AminesBenzenesulphonyl chloride does not react with Allylamine N N Diethylbutylamine

Organic Chemistry
Biomolecules40 Which of the following properties of glucose cannot be explained by its open chain structure i Glucose does not form hydrogen sulphite with NaHSO ii On oxidation with HNO3 glucose gives saccharic acid iii Glucose is found to exist in two different crystalline forms which are named as a and B a ii only b i and iii c ii and iii d i and ii uiches proline from natural a amino acids

Organic Chemistry
General organic chemistryWhich of the following choices represent s a pair of resonance forms Mark all that apply 0 0 11 CH CH CH H H w propere OH CH CH CH H C CN H CH CH OCH CH CH OCH onance

Organic Chemistry
Chemistry in Daily LifeCompound 1 is hard to afford but it is possible to synthesis 1 as shown below CI Rot CN Lok 2 propanol OK HN NH 1 1 What is the proper name of the compound 1 Also what is the name that most commonly appears as a in consumer products

Organic Chemistry
Biomolecules1 Which substance has the weakest attractions between its molecules Oformic acid O benzene O castor oil Ophenol Substance Melting Point C castor oil phenol benzene formic acid 9 8 43 2 5 7 8 K

Organic Chemistry
General organic chemistryWhat are the products of phospholipase D degradation of phosphatidylcholine O A Phosphatidic acid phosphatidate and choline O B Diacylglycerol and phosphoethanolamine O C Phosphatidic acid phosphatidate and ethanolamine O D Diacylglycerol and phosphocholine

Organic Chemistry
General organic chemistry1 216 g Organic compound analysed by kjeldahl methodand NH3 evolved was absorbed in 100ml N H2SO4 Remaining solution was made upto 500ml by water 20 ml of this solution require 32ml N 10 NaOH for complete neutralisation Find N

Organic Chemistry
Aldehydes & KetonesAcetaldehyde is treated with excess of methanol in presence of HCI gas The final major product obtained is CH O CH C H OCH3 CH3 CHOCH3 CH3 C OCH3 oc CH3 CH OH OCH3

Organic Chemistry
General organic chemistryIn case of optically active alkyl halides SN1 reactions are accompanied by racemisation Can you think of the reason why it happens Actually the carbocation formed in the slow step being sp ybridised is planar achiral The attack of the nucleophile may be accomplished from either side of the plane of carbocation resulting in mixture of products one having the same configuration the OH

Organic Chemistry
Aldehydes & Ketonesere ochemistry major product formed in each step for each of the following reactions Please give an explanation for your answer BzO S a Chx BCI Me NEt CH Cl 78 C b CH3CH CHO 78 to 25 C c H O2 NaOH workup A 82 ds 95 5

Organic Chemistry
General organic chemistryQuestion No 85 Single Correct A HCN KOH product B is A OH A CHO OH NH OH X OH CH NH i LIAH Bi ii H O CN A

Organic Chemistry
IsomerismWhich of the following is are incorrect IUPAC name a 4 chloro 3 methyl cyclopentanol 1 b 1 amino 3 bromo hexan 1 one c 4 chloro 3 methyl cyclohexane carboxylic acid d 3 bromo 1 methyl hexan 1 ol Key A B D

Organic Chemistry
General organic chemistry81 180 Consider the following compounds CHO 1 ii iii keto enol tautomerism is observed in i and ii only i only iii only i and iii only B

Organic Chemistry
Aldehydes & KetonesOne mole of C6H5COCH CH3 is treated with one mole of Br in basic solution the product s formed is are A B C 1 mole of C H5COCB CH3 0 5mole of C H5COCB CH3 1 mol of C H5COCHBr CH Br

Organic Chemistry
Practical DetectionRupa took 3 mL of sodium sulphate solution in a test tube and added about 3 mL of barium chloride solution to it She observed hat The solution turned black O Red precipitate was formed O White precipitate was formed The solution turned red

Organic Chemistry
General organic chemistryO The Fischer projection formula that represents the following compound is A C CH H OH H OH HO H CH 3 OH H CH3 H OH H CH3 OH H C OH H OH H OH CH3 B D OH H CH3 HO H H OH CH3 HO HO H CH 3 H H CH3 OH

Organic Chemistry
AminesConsider the following reaction sequence CHO HNO H SO 273 283 K Product B is CH NHNH NO 1 CH NH CH NO NO A CH OH i NH NH ii KOH CH CH A OH OH B

Organic Chemistry
Hydrocarbons2 Three isomeric compounds M N and P CsH 00 give the following tests i M and P react with sodium bisulfite to form an adduct ii N consumes 1 mol of bromine and also gives turbidity with conc HCl anhydrous ZnCl after prolong heating iii M reacts with excess of iodine in alkaline solution to give yellow crystalline compound with a characteristic smell iv p Rosaniline treated with sulphur dioxide develops pink colour on shaking with P The structures of M N and P respectively are A B C D M OH OH N OH e e OH P H ito D

Organic Chemistry
Aldehydes & Ketones1 i H C i CO ii H O H i C6H CH CuLi A H C B H C C H C D H C O Identify D in the following sequence of reaction CH3 CH3 Br CH O B D O CH3 CH3 C CH Ph O Mg Ether CH3 HOS A C Ph CH3 SOCI 2 C C CH Ph A C CH Ph 02 0

Organic Chemistry
Reactions of benzene0 52 Match List I with List II List I F P R S NO2 Br Cl O S O CF3 NO CF3 KSH A COO NaNH liq NH3 A R NH A 5 1 2 3 4 List II 1 Addition elimination reaction 2 Elimination addition Reaction 3 SNAr takes place 4 Benzyne intermediate

Organic Chemistry
Chemistry in Daily Life2 An unknown organic molecule of C4H6Cl4 is characterized by having the following chemical shifts 8 3 9 ppm doublet 4H 8 4 6 ppm triplet 2H Identify the structure of the unknown compound show full work

Organic Chemistry
Reactions of benzeneWhen naphthalene undergoes an irreversible electrophilic aromatic substitution the major product is the kinetic product which proceeds through the most stable arenium ion intermediate With this in mind draw the curved arrow mechanism for the first step of the electrophilic aromatic substitution of naphthalene with the acylium ion generated from acetyl chloride and AICI Then draw the curved arrows and a resonance structure in step 2 with an unbroken benzenoid ring Draw all atoms electrons and charges if necessary on all structures draw curved arrows on all structures except the last one Do not draw any inorganic byproducts or counterions

Organic Chemistry
General organic chemistryStatement 1 RCOCI RCO 0 and RCOOR all react with Grignard reagents to form 3 alcohols Statement 2 RCOCI reacts with R Cd to form ketones but RCO 0 and RCOOR do not react at all A B Both Statement 1 and Statement 2 are true and Statement 2 is the correct explanation of Statement 1 Both Statement 1 and Statement 2 are true and Statement 2 is not the correct explanation of

Organic Chemistry
General organic chemistry3 Which of the following is the correct order C C bond lengths among these compounds 1 CH O CH CH NO II CH CH NO III CH CH CH IV CH CH 1 I II II IV 2 IV III II I

Organic Chemistry
IsomerismNO Single Correct A Which of the following show tautomerism NO b a CH CH NO CH CH C CHCCHO CH A a d d A Da CH

Organic Chemistry
Carboxylic acids71 72 73 74 75 76 77 78 79 80 81 4 X 1 Identify the product CH 1 CH CH Question Type Single Correct Type 2 3 COOH CHO CHO COOH COOH COOH COOLE English Prev acidified product KMnO t Review Q