Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
AminesWhich of the following is the first step in the Gabriel synthesis of primary amines?
Deprotonation of phthalimide.
Nucleophilic substitution.
Hydrolysis of the phthalimide derivative.
Deprotonation of the ammonium salt.

Organic Chemistry
General organic chemistryIdeal Gas Law Practice Problems
Use the ideal gas law, PV = nRT and the universal gas law constant to solve the
following problems. Remember your temperature must be in Kelvin and round your final
answer to significant figures.
If pressure unit is in atm, use:
If pressure unit is kPa, use:
R = 0.0821 L x atm / K x mol
R = 8.31 kPax L K x mol
1. If I have 3 moles of a gas at pressure of 2.3 atm and a volume of 11 liters, what is
the temperature?
2. If I have a gas at a pressure of 1.4 atm, a volume of 38 liters and a temperature of 84
degrees Celsius, how many moles of gas do I have?

Organic Chemistry
General organic chemistryUse the ideal gas law, PV = nRT and the universal gas law constant to solve the
following problems. Remember your temperature must be in Kelvin and round your final
answer to significant figures.
If pressure unit is in atm, use:
If pressure unit is kPa, use:
R = 0.0821 L x atm /Kx mol
R = 8.31 kPax L K x mol
1. If I have 3 moles of a gas at pressure of 2.3 atm and a volume of 11 liters, what is
the temperature?

Organic Chemistry
General organic chemistry18.33 Draw both products that are obtained when 4-chloro-2-methyltoluene is treated with NaNH, followed by treatment with H₂O*.
18.34 Starting with benzene and using any other necessary reagents of your hair

Organic Chemistry
General organic chemistryThe lines in the line spectrum of an atom results from
energy absorbed by electrons dropping back down to a lower energy level
energy absorbed by electrons jumping to a higher energy level
energy released by electrons jumping to a higher energy level
energy released by electrons dropping back down to a lower energy level
none of the above

Organic Chemistry
General organic chemistryA gas mixture contains 237 kPa of sulfur dioxide and 125 kPa of carbon dioxide.
What is the total pressure of the mixture?
Select the correct answer below:
2.96 x 104 kPa
237 kPa
112 kPa
362 kPa

Organic Chemistry
Practical DetectionAccording to VSEPR theory, molecules adjust their shapes to keep which of the
following as far apart as possible?
pairs of valence electrons
inner shell electrons
bonding pairs of electrons
mobile electrons
lone pairs of electrons

Organic Chemistry
Chemistry in Daily LifeA cube of aluminum at 15.4 °C is submerged into water that is 98.2 °C. In this
situation, heat will be transferred from the aluminum to the water.
True
False

Organic Chemistry
Chemistry in Daily LifeAn expandable container contains 1.25 grams of helium. What is its volume (in L)
when it has a pressure of 0.955 atm and a temperature of 22°C?
Report your answer to the correct number of significant figures. Show your work
here or on a separate sheet of paper to receive full credit for your answer.

Organic Chemistry
General organic chemistryIntermolecular forces are
Forces within covalent molecules that hold them together
Electrostatic forces between ions
Bonds between hydrogen and oxygen atoms in water molecules
Attractive forces between separate molecules
Covalent bonds within a network solid

Organic Chemistry
Chemistry in Daily LifeWhat does the term "dry" gas pressure refer to when a gas sample is collected over
water?
Select the correct answer below:
Atmospheric pressure
Vapor pressure of water
Total pressure of the pure gas plus the water vapor pressure
Pressure of the pure gas

Organic Chemistry
General organic chemistryWhat made scientists first believe that atoms contain equal numbers of protons and
electrons?
most alpha particles went straight through the gold foil
some alpha particles were deflected by the gold foil
the line spectra of excited atoms
atoms are electrically neutral
two of the above

Organic Chemistry
General organic chemistryFor this question you will need to draw the VSEPR diagram of CH₂O. You will also
need to list the number of bonding pairs and lone pairs on the central atom as well as
including the name of the shape of the molecule. You need to show all of your work.
This includes the valence electrons calculations, and drawings of what the molecules
looks like as you are adding electrons and creating bonds. You should include at least
3 diagrams showing the progression of your molecule. Please take a picture of your
drawings and calculations and upload it to the "Unit 1 - Test" dropbox within 5
minutes of finishing the test.

Organic Chemistry
General organic chemistryA sample of oxygen collected over water at a temperature of 29.0 °C exerts a
pressure of 764 torr has a volume of 0.560 L. What is the dry gas pressure of the
oxygen? (The vapor pressure of water at 29.0 °C is 30 torr.)
Select the correct answer below:
730 torr
728 torr
734 torr
740 torr
9

Organic Chemistry
Practical DetectionWhich of the following is the electron configuration for aluminum in the ground
state? (The numbers after the letters are meant to be superscript)
1s2 2s2 2p5 3s1 3p1
1s2 2s2 2p6 3s2 3p1
1s2 2s2 2p6 3s1 3p2
1s2 2s2 2p6 3s1 3p1
1s2 2s2 2p6

Organic Chemistry
Practical Detection12. Marketing people typically have the following basic objectives to accomplish. Which one below is
not one of them:
a. Focus only on developing new markets.
b. Maximize the sales of existing products in existing markets.
c. Develop and sell new products.
d. Provide the quality of service necessary for customers to be satisfied with their transactions and to
continue doing business with the organization.

Organic Chemistry
General organic chemistryWhat do the 3s and 3p orbitals in the hydrogen atom have in common? Select all
that apply.
They are in the same principal shell.
They are in the same subshell.
They have the same energy.
They have the same basic shape.

Organic Chemistry
General organic chemistryWhich was an assumption Bohr made in his model?
Select the correct answer below:
Wavelengths have negative values.
Energy values were quantized.
Neutrons are negatively charged.
Electrons are found in the nucleus.

Organic Chemistry
General organic chemistry6
#1 - List the 3 types of intramolecular forces. Place the forces in order from strongest to weakest (I.e., #1 is the strongest; #3 is the weakest).
3 pts
#2 - List the 3 types of intermolecular forces.
3 pts
#3 - Convert the following (show all math work for conversion): 847 mm Hg = ? kPa
5 pts

Organic Chemistry
Chemistry in Daily LifeWhich does NOT influence the bond dipole moment?
Select the correct answer below:
The electronegativity difference between the atoms in the bond.
The magnitude of the partial charges on the atoms in the bond.
The distance between the atoms in the bond.
The bond dissociation energy of the bond.

Organic Chemistry
General organic chemistryA molecule has 9 valence electrons. What is true about its Lewis structure?
Select the correct answer below:
It will follow the octet rule.
It will be an exception to the octet le because it is an odd electron
molecule.
It will be an exception to the octet rule because it has an electron deficient
central atom.
It will be an exception to the octet rule because it is hypervalent.

Organic Chemistry
Practical Detection6. The following characteristic applies to which of the eight characteristics of success of sales: They
can match up their product's benefits with the customer's needs.
a. Use the Golden Rule of Selling
b. Sales knowledge at M.D. level
c. Excels at Strategic thinking
d. Stamina for the challenge

Organic Chemistry
Practical DetectionWhat did classical physics predict about the structure of the atom?
Select the correct answer below:
Based on electrostatic attraction, the electrons are repelled by the protons in
the nucleus.
Based on electrostatic attraction, the electrons would spiral into the nucleus.
Based on electrostatic attraction, the electrons were attracted to other
electrons.
Based on electrostatic attraction, atoms are inherently stable.

Organic Chemistry
General organic chemistryWhat is the electron-pair geometry and molecular structure of ammonia (NH₂)?
Select the correct answer below:
tetrahedral, tetrahedral
tetrahedral, trigonal pyramidal
trigonal pyramidal, tetrahedral
trigonal pyramidal, bent

Organic Chemistry
General organic chemistryA molecule has a central atom from the fourth period. The central atom is bonded
to five other atoms. What is true about this molecule's Lewis structure?
Select the correct answer below:
It will follow the octet rule.
It will be an exception to the octet rule because it is an odd electron
molecule.
It will be an exception to the octet rule because it has an electron deficient
central atom.
It will be an exception to the octet rule because it is hypervalent.

Organic Chemistry
General organic chemistryCalculate the grams of aluminum hydroxide that will be produced if 86.865 grams of ammonium hydroxide react. Use the balanced
chemical equation below.
Al2(SO4)3(s) + 6NH4OH(aq) → 2Al(OH)3(s) + 3(NH4)₂SO4(aq)
Write your answer with the correct number of significant figures, units and chemical formula.

Organic Chemistry
HydrocarbonsIdentify the type of transport described by each of the following:
a. an ion moves from low to high concentration in the cell.
b. carbon dioxide moves through a cell membrane.

Organic Chemistry
Chemistry in Daily Life16.Indicate whether each of the following statements describes primary, secondary,
tertiary, or quaternary protein structure:
a. Hydrophobic R groups seeking a nonpolar environment move toward the inside of
the folded protein.
b. An active protein contains four tertiary subunits.
c. Valine replaces glutamate in the beta-chain.
d. Protein chains of collagen form a triple helix.

Organic Chemistry
General organic chemistry.Draw the condensed structural formulas for each of the following peptides and give the
three-letter and/or one-letter abbreviations:
a. prolylaspartate
b. threonylleucine
c. methionylglutaminyllysine
d. histidylglycylglutamylisoleucine

Organic Chemistry
HydrocarbonsWhich of the following statements are true?
Select all that apply:
Covalent bonding forms as a result of a transfer of electrons from one atom
to another.
Covalent bonding forms when two atoms share electrons between them.
Covalent bonding typically forms between two metal atoms.
Covalent bonding typically forms between two nonmetal atoms.

Organic Chemistry
General organic chemistryUsing the Frost circle method (polygon method) provide an energy_diagram showing the relative energies of the molecular
orbitals for the cyclopentadienyl anion. Clearly label and number each molecular orbital as bonding, nonbonding, antibonding (use
*). Clearly show the nonbonding line and the ground state electron configuration.

Organic Chemistry
BiomoleculesDraw an example of each type of the following lipids:
a. Fatty acid (saturated and unsaturated)
b. Prostaglandin
c. Wax
d. Triacylglycerol (Fat and oil)
e. Glycerophospholipid
f. Sphingolipid
g. Steroid

Organic Chemistry
General organic chemistryA 150-ml bottle of mouthwash contains 14 mL of ethanol. What is the volume/volume percent
concentration of ethanol?
% (v/v) ethanol

Organic Chemistry
General organic chemistryWhat does the equation represent?
.Ca. → Ca²+ + 2e¯
Select the correct answer below:
Anions are formed by gaining valence electrons.
Anions are formed by losing valence electrons.
Cations are formed by gaining valence electrons.
Cations are formed by losing valence electrons.

Organic Chemistry
Chemistry in Daily LifeHow many milliliters of a 5.2 M NaCl solution would be needed to prepare each solution?
a. 15 mL of a 0.17 M solution:
b. 350 mL of a 0.039 M solution:
mL
mL

Organic Chemistry
General organic chemistryHund's rule states that the electron configuration with the lowest-energy will have the
maximum possible number of unpaired electrons. Which of the elements below
would require special attention to this rule to correctly depict the orbital diagram?
Select the correct answer below:
H
He
N
Be
E

Organic Chemistry
General organic chemistryCalculate the molarity of each aqueous solution with the given amount of NaCl (molar mass
58.44 g/mol) and final volume.
a. 2.60 mol in 0.920 L
M
b. 5.90 mol in 730. mL
M
c. 0.0920 mol in 8.90 mL
M

Organic Chemistry
AminesDraw the condensed structural formula for the four amine isomers with the formula C3HgN. Write the common name and indicate whether they are primary, 1°, secondary, 2°, or tertiary, 3°, amines.

Organic Chemistry
Chemistry in Daily LifeA chemist placed a 10.00 mL sample of HCI in a flask. The HCI was titrated with 0.100
M NaOH. The titration required 22.08 mL of NaOH to reach the equivalence point.
What is the molarity of the HCI sample?
0.0221 M
0.00221 M
0.0453 M
0.221 M

Organic Chemistry
General organic chemistryEnter your answer in the provided box.
What is the weight/volume percent concentration using the given amount of solute and total volume of
solution?
67 g of NaNO3 in 250 mL of solution:
% (w/v) NaNO3

Organic Chemistry
Practical Detection3. Identify the limiting reactant and the theoretical yield of phosphorous acid if 3.00 mol
of PCl3 is mixed with 3.00 mol of water.
vnnnnn
PC13 + H₂O
H3PO, +
HCI
4. In number 3, how much of the excess reactant is left over?
What is the percent yield if only 58.7 g of H3PO3 is created?

Organic Chemistry
General organic chemistryBe sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 36.0 g of NaCl in 680 mL of solution:
b. 46.0 g of NaHCO3 in 8.8 L of solution:

Organic Chemistry
General organic chemistryCircle and identify the functional groups in the following molecules...each molecule
has 2 functional groups. (2 pts)

Organic Chemistry
General organic chemistryIf the solubility of KCI in 100 mL of H₂O is 34 g at 20°C and 43 g at 50°C, label each of the following
solutions as unsaturated, saturated, or supersaturated. If more solid is added than can dissolve in the
solvent, assume that undissolved solid remains at the bottom of the flask.
a. adding 14 g to 100 mL of H₂O at 20°C: (select)
b. adding 42 g to 100 mL of H₂O at 50°C: (select)
c. adding 17 g to 50 mL of H₂O at 20°C: (select)
d. adding 38 g to 100 mL of H₂O at 50°C and slowly cooling to 20°C to give a clear solution with no
precipitate: (select)

Organic Chemistry
General organic chemistryBe sure to answer all parts.
What is the weight/volume percent concentration of a 31.0 % (w/v) solution of vitamin C after each of
the following dilutions?
a. 170. mL diluted to 490. mL:
b. 0.39 L diluted to 860 mL:

Organic Chemistry
General organic chemistryBe sure to answer all parts.
How many grams of NaCl are contained in each of the following volumes of a 1.23 M solution?
b. 62 mL
a. 0.24 L
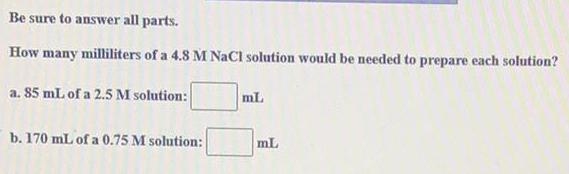
Organic Chemistry
Chemistry in Daily LifeBe sure to answer all parts.
How many milliliters of a 4.8 M NaCl solution would be needed to prepare each solution?
a. 85 mL of a 2.5 M solution:
b. 170 mL of a 0.75 M solution:
mL
mL
![Be sure to answer all parts.
One gram (1.00 g) of vitamin B3 (niacin) is dissolved in water to give 15.0 mL of solution. (a) What is
the weight/volume percent concentration of this solution? (b) What is the concentration of a solution
formed by diluting 1.0 mL of this solution to each of the following volumes: [1] 16.0 mL; [2] 120 mL?](https://media.kunduz.com/media/sug-question/raw/52961263-1658984128.663357.jpeg?w=256)
Organic Chemistry
General organic chemistryBe sure to answer all parts.
One gram (1.00 g) of vitamin B3 (niacin) is dissolved in water to give 15.0 mL of solution. (a) What is
the weight/volume percent concentration of this solution? (b) What is the concentration of a solution
formed by diluting 1.0 mL of this solution to each of the following volumes: [1] 16.0 mL; [2] 120 mL?

Organic Chemistry
Practical DetectionA drink sold in a health food store contains 2.50% (w/v) of vitamin C. What volume would you have to
ingest to obtain 1,000. mg of vitamin C?
mL solution

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
If a solution contains 240. mEq/L of Na*, how many milliequivalents of Nat are present in 440. mL of
solution?