Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibrium21 What is NH in a solution that is 0 02 M NH and 0 01 M KOH K NH 1 8 105 1 3 6 x 10 5 M 2 1 8 x 105 M 3 0 9 x 10 5 M 4 7 2 x 10 5 M

Physical Chemistry
GeneralD The pressure in a vessel that contained pure oxygen dropped from 2000 torr to 1500 torr in 55 minute as the oxygen leaked through a small hole into a vacuum When the same vessel is filled with another gas the pressure dropped from 2000 torr to 1500 torr in 85 minute What is the molecular weight of gas

Physical Chemistry
Electrochemistry1 2 A big irregular shaped vessel contained water the conductivity of which was 2 56 x 10 5 S cm 500 g of NaCl was then added to the water and the conductivity after the addition o NaCl was found to be 3 10 x 105 S cm The capacity c the vessel if it is fulfilled with water A of NaCl 149 9 baris m 0 Hand Toil 105 litres or c 2467 5 x 102 litres d 2 3725 105 litres a 4587 9 102 litres b 3 4752 x

Physical Chemistry
Chemical BondingThree moles of an ideal gas are taken through a cyclic process ABCA as shown on T V diagram in the figure The gas loses 2510 J of heat in the complete cycle If TA 100 K and TB 200 K The work done by the gas during the process BC is R 8 3 J mol K Tel Te TA 1 VA Vc O 5000 J O 5000J O 4000 J VB

Physical Chemistry
ElectrochemistryFor which of these oxidation reduction pairs will the reduction potential vary with pH 4 II AmO2 Am I AmO AmO III Am Am 1 I only 3 I and II only 2 II only 4 I II and III

Physical Chemistry
ElectrochemistryConsidering the elements F Cl O and N the correct order of their chemical reactivity in terms of oxidizing property is a F Cl O N c Cl F O N b F O Cl N d O F N Cl

Physical Chemistry
GeneralIf C and C denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively then JAYC C R 28 B Cp C R 14 C C C R D Cp C 28 R refrigetator If the work done

Physical Chemistry
GeneralN 6 022 x1023 hydroxide OH sulfate SO4 Calculate the mass in grams of 3 25 x1023 molecules of trinitrogen tetraoxide

Physical Chemistry
General2 Calculate the isothermal 1 av ideal gas a V ap gas at T 273K compressibility a for a at P 1 bar and cub T n expansion coefficient 3 P n 1 JP for an ide

Physical Chemistry
GeneralWhich of the following aqueous solution will 72 result in maximum number of moles of metal deposited on passing same charge 1 KI 2 ZnSO4 3 AuCl3 4 CaCl

Physical Chemistry
GeneralQ39 How many grams of methyl alcohol should be added to 10 litre tank of water to prevent it from freezing at 298K K for water is 1 86 K Kg mol A 880 07g B 899 04g C 886 02g D 868 06 g

Physical Chemistry
GeneralWhen ethene reacts with bromine in aqueous sodium chloride Solution The product s obtained is are 1 Ethylene dibromide only 2 Ethylene dibromide and 1 bromo 2 chloro ethane 3 1 bromo 2 chloroethane only 4 Ethylene dichloride only CH CH CA

Physical Chemistry
GeneralWhich of the following statement is true a 6 electrons are present in Mg for which m 0 b 6 electron are present in one p orbital of Mg c Maximum 18 electrons present in M shell 12 d 3 electron present in phosphorous for which 0 m 0 s A a b c d B a be C a c d D c

Physical Chemistry
Generalc NH3 d SF6 149 Under which of the following sets of conditions is a real gas expected to deviate from ideal behaviour I High pressure small volume II High temperature low pressure III Low temperature high pressure b only II PV nRT if pre vu so then expresia c only III const ideal gas d I and III both chaviour a plot between D D was

Physical Chemistry
Gaseous and liquid statesInitially in a container 1 g of gas A has 4 atm pressure at constant temperature If 2 g of gas B is added in same container at same temperature then pressure becomes 6 atm what will be the ratio of molecular weight of A and B 1 M 4MB A 3 M 2M S 2 M 2MB A 4 MB 4MA D

Physical Chemistry
General3 1 53 x 10 mol A transition metal M forms a volatile chloride which chlorine the formula of the metal chloride will be 1 MC1 2 MC4 4 4 46 x 10 mol has a vapour density of 94 8 If it contains 74 75 AIEEE ONLINE 2015 4 MC13 3 MC15

Physical Chemistry
EquilibriumThe activation energies for the forward and reverse elementary reactions in the system A B are 10 303 and 8 000 kcal respectively at 500 K Assuming the pre exponential factor to be the same for both the forward and reverse steps the equilibrium constant of the reaction at 500 K is a 1 00 c 100 b 10 0 d 0 1

Physical Chemistry
Atomic StructureThe ground state energy of hydrogen atom is 13 6 eV Consider an electronic state of He whose energy azi muthal quantum number and magnetic quantum number are 3 4 eV 2 and 0 respectively Which of the following statement s is are true for the state A It is a 4d state B It has 2 angular nodes C It has 3 radial nodes D The nuclear charge experienced by the electron in this state is less than 2e where e is the magnitude ose of the electronic charge

Physical Chemistry
Nuclear chemistry6 The minimum energy required for the emission of photoelectron from the surface of a metal is 4 95 10 9 J Calculate the critical frequency and the corresponding wavelength of the photon required to eject the electron h 6 6x 10 34 J sec

Physical Chemistry
ElectrochemistryThe specific conductance of a saturated AgCl solution is found to be 1 86 x 10 6 Scm and th for pure water is 6 0 x 10 8 S cm If for AgCl is 137 2 Scm eqvt the solubility of AgCl water would be A 1 7 x 103 M C 1 3 x 10 4 M B 1 3 x 10 5 M D 1 3 x 10 M A magnetic moment of 1 73 B M will be shown by one of the following compounds

Physical Chemistry
GeneralH Cl reaction heat of formation of HCI in kJ is a 194 kJ b 97 kJ c 97 kJ d 194 k 2 HCI AH 194 kJ In thi

Physical Chemistry
Solid state0 If the length of the unit cell is 54 the smallest 0 distance in between the two neighbouring A metal atoms in a face centred cubic lattice is 112 50 215 3 7 07 EAM 2011 4 3 535

Physical Chemistry
Gaseous and liquid statesthis IIT 1993 7 The average velocity of gas molecules is 400 m sec Calculate its rms velocity at the same temperature DUT 20031

Physical Chemistry
Atomic StructureWhich of the following shapes of SF4 is more stable and why On F S F i F F S F ii F F

Physical Chemistry
Equilibrium109 A definite amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0 1 atm pressure NH4HS decomposes to give NH3 and H S and at equilibrium total pressure in flask is 1 1 atm If the equilibrium constant K for the reaction NH4HS s NH g H S g is represented as zx 10 then find the value of z 3

Physical Chemistry
GeneralPaper chromatography is an example of 1 Partition chromatography Thin layer chromatography 2 3 Column chromatography tion chromatography

Physical Chemistry
Generala 1800 23 2 If mass of one atom is 3 32 x 10g then calculate number of nucleons neutrons and protons present in 2 atoms of the element a 40 b 20 c 10 rent in 9 5 g of PO d 40 NA

Physical Chemistry
SolutionsAn ideal solution was obtained by mixing methanol and ethanol If the partial vapour pressure of methanol and ethanol are 2 619 K Pa and 4 556 K Pa respectively the composition of vapour in terms of mole fraction will be 1 0 635 MeOH 0 365 EtOH 2 0 365 MeOH 0 635 EtOH 3 0 574 MeOH 0 326 EtOH 4 0 173 MeOH 0 827 EtOH

Physical Chemistry
Electrochemistry6 The standard potentials at 25 C for the following half cell reactions are given as 2 Zn 2e Zn E 0 762 V esibi 2 Mg 2e Mg E 2 37 V cell When zinc dust is added to a solution of magnesium chloride A no reaction will take place B zinc chloride is formed C zinc dissolve in solution D magnesium is precipitated

Physical Chemistry
GeneralWeight of Agrobtained passed for TO0 second 1 0 11 g 3 10 g when 1 ampere current is in Ag ion solution 2 1 08 g 4 0 05 g

Physical Chemistry
Chemical kinetics10 ml of methyl acetate was taken in a flask containing 200 ml of 0 1 N HCI maintained at 30 C 20 ml o the reaction mixture was taken at different intervals of time and titrated with a standard alkali The following data were obtained Time minutes Alkali used ml The order of reaction is Zero order Second order 0 19 24 75 24 24 119 26 60 183 29 32 2 First order 4 Third order 80 42 03

Physical Chemistry
Solid state6 Body diagonal of a cube is 866 pm Its edge length would be 1 408 pm 3 500 pm 2 1000 pm 4 600 pm

Physical Chemistry
Chemical kinetics4 7 For the reaction N O g 2NO g it has been found that the pressure of N O4 falls from 0 64 atm to 0 38 atm in 28 minutes Calculate the rate of reaction and the rate of appearance of NO g
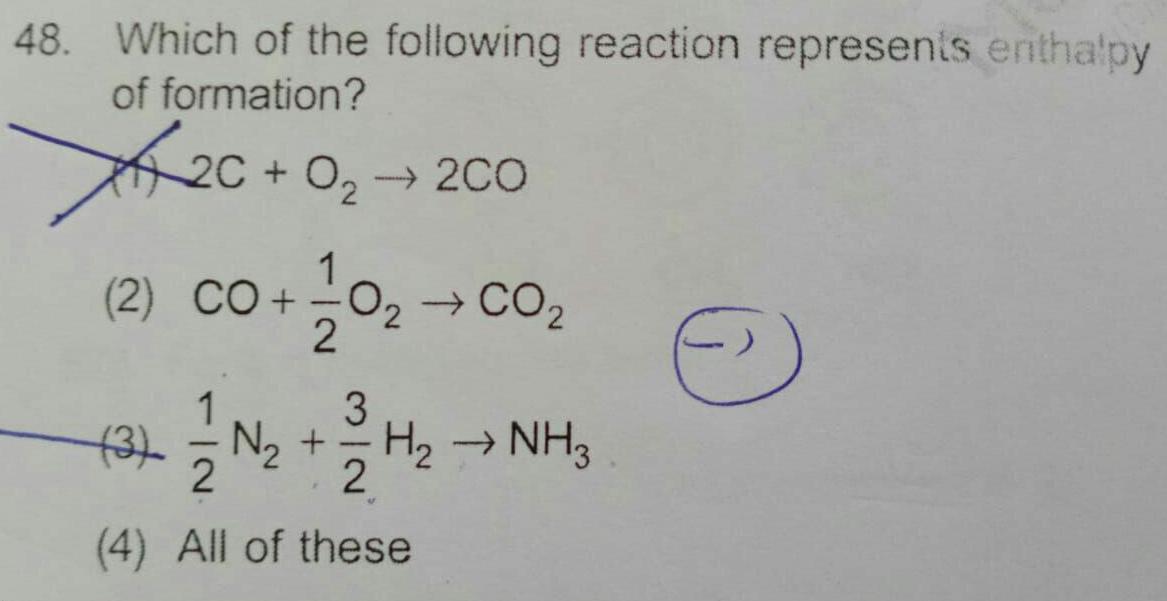
Physical Chemistry
General48 Which of the following reaction represents enthalpy of formation 0 2C 0 200 1 2 CO 0 CO 2 1 3 3 N H NH3 2 4 All of these

Physical Chemistry
General2 In the process of rusting of iron the weight of oxygen that reacts with 5 6 g of iron is A 1 2g B 2 4 g C 3 6 g D 4 8 g

Physical Chemistry
GeneralOne litre of N 2 HCl solution was heated in a beaker When volume was reduced to 600 mL 3 25 g of HC was given out The new normality of solution is a 6 85 c 0 1043 b 0 685 d 6 50

Physical Chemistry
Electrochemistry1 Half cell reactions for some electrodes are given below 1 A e A E 0 96 V II B e B E 0 12 V III C e E 0 18 V IV D 2e D E 1 12 V Largest potential will be generated in which cell C 1 A A B B 3 B B C C 2 DD2 A A 4 DID 1 1C

Physical Chemistry
General10 ml of a gaseous compound containing N and O is mixed with 30 ml of H to produce H O and 10 of N g Molecular formula of compound if both reactants reacts completely is A NO C N O3 B NO D N O5

Physical Chemistry
ElectrochemistryThe ionic conductance of Ba2 and Cl respec tively are 127 and 7692 cm at infinite dilution The equivalent conductance of BaCl at infinite dilution will be a 33052 cm c 13992 cm b 20352 cm d 512 cm

Physical Chemistry
EquilibriumWhich of the following combinations would not result in the formation of a buffer solution a NH HCI b NH C1 NH c CH COOH NaCl d NaOH CH COOH

Physical Chemistry
General3 Th 234 disintegrates and emits 63 and 7a particles to form a stable product Find the atomic number and mass number of the stable product and also identify the element IIT 2004

Physical Chemistry
GeneralWhat is the weight of sodium bromate and molarit of solution to prepare 85 5 mL of 0 672 N solution when half cell reaction are i BrO3 6H 6e 10e ii 2BrO3 12H Br 3H O Br 6H 0

Physical Chemistry
ElectrochemistryA direct current deposits 54 g of silver atomic mass 108 during the electrolysis The same quantity of electricity would deposit aluminium atomic mass 27 from aluminium chloride in molten state equal to 2 5 4 g 4 27 g 1 4 5 g 3 54 g
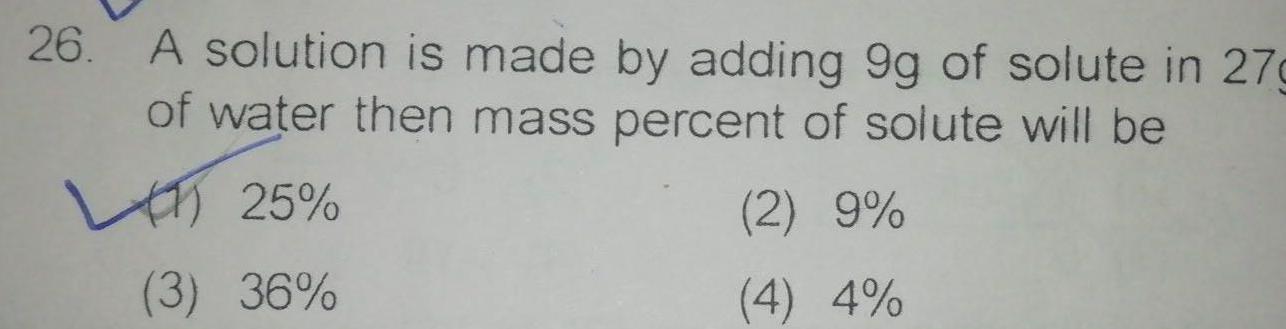
Physical Chemistry
General26 A solution is made by adding 9g of solute in 27g of water then mass percent of solute will be 25 3 36 2 9 4 4

Physical Chemistry
Solid stateA compound forms hexagonal close packed structure What is the total number of voids in 0 5 mol of it 0 1640 1 9 1023 3 3 10 3 2 6 10 3 4 1 8 10 3 ing blond

Physical Chemistry
GeneralCalculate the pH of 1 x 10 solution of NH4OH The dissociation constant of NH4OH is 1 85 10 5 mol dm Sol Degree of dissociation a 1 85 10 5 1 10 3 1 36 10 1 Degree of dissociation represents the But V c C concentrations of OH in the solution OH 1 36 10 1 Kb K H O JOH 1 1 85 10 2

Physical Chemistry
Surface chemistryCalculate the volume at 300 K at 1 atm and amount adsorbed per g of 2g solid surface if 4g of N is allowed to adsorbed at 300 K and 0 8 atm 1 2 5 litre g 2 3 2 litre g 3 1 76 litre g 4 5 62 litre g

Physical Chemistry
GeneralA monoatomic ideal gas undergoes a process in which the ratio of p to Vat any instant is constant and equals to 1 What is he molar heat capacity of the gas 2006 3M 3R 5R 4R 2 b 2 c d 0

Physical Chemistry
Chemical kinetics4 For given reaction 2Bt aq A s 2B s A 2 If Keq of reaction is 1015 2 and E A 2 A 0 34V DA Then calculate E B B will be 1 0 80 loe onse 2 0 80 4 1 60 27

Physical Chemistry
Surface chemistry58 X 1 501 5 50 Which mixture of the solutions will lead to the formation of negatively charged colloidal AgI Isol a 50 of 0 1 M AgNO3 50 mL of 0 1 MKT b 50 mL of 1 M AgNO3 50 mL of 1 5 M KI c 50 mL of 1 M AgNO3 50 mL of 2 M KI d 50 ml LO 5