General Questions and Answers

Physical Chemistry
GeneralWhat is the identity of a metal that has a mass of 27 0 g and a volume of 10 0 cm O Lead Aluminum Copper Gold

Physical Chemistry
GeneralIf you do a lab that burns Magnesium in pure Oxygen what would the empirical formula of the compound be if you started with 0 6 g Magnesium and 0 4 g of Oxygen O Mg20 Mg302 O MgO Mg203


Physical Chemistry
General7 What mRNA base sequence is the complementary daughter strand to the following DNA informational parent strand Write the sequence of amino acids 5 AAUGAUAGGCCUCCGGCAUGA 3

Physical Chemistry
GeneralHow many grams of sodium chloride NaCl should be added to prepare 50 0mL of a 2 50 M NaCl NaCl 58 443 g mol
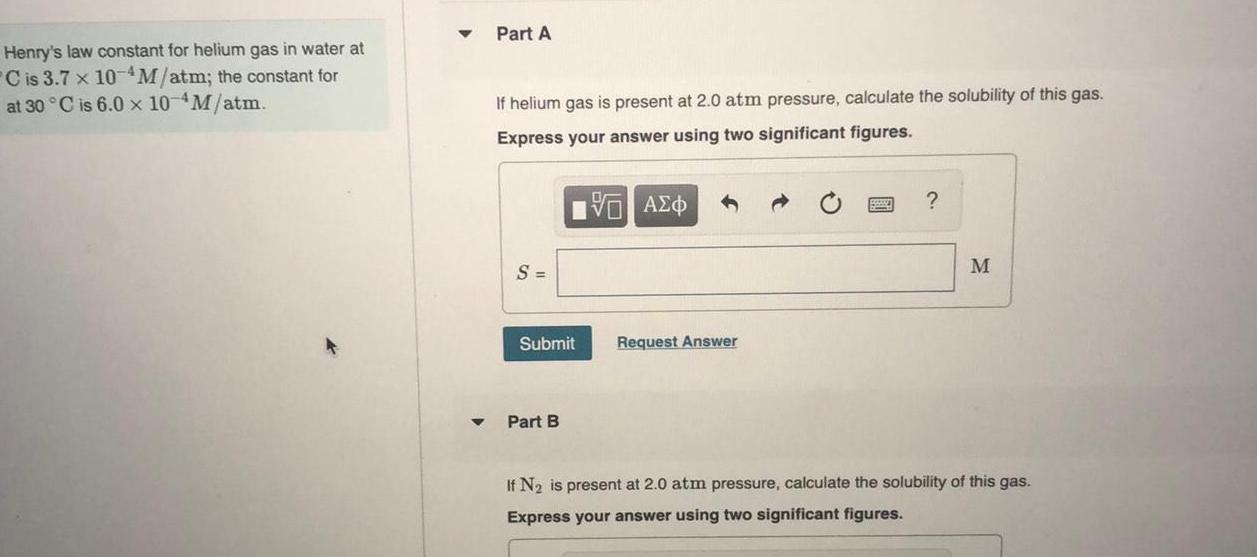
Physical Chemistry
GeneralHenry s law constant for helium gas in water at C is 3 7 x 10 M atm the constant for at 30 C is 6 0 x 10 M atm Part A If helium gas is present at 2 0 atm pressure calculate the solubility of this gas Express your answer using two significant figures S Submit Part B VAEO Request Answer M If N is present at 2 0 atm pressure calculate the solubility of this gas Express your answer using two significant figures

Physical Chemistry
GeneralStudents in a lab are doing experiments involving the motion of objects They pull an object across a table and use a detector to measure the acceleration of the object for the entire time they pull it The graph shows the data from the detector with four segments labeled Acceleration 3 Time For which segments of the motion is there a net force on the object Explain how the graph indicates this Enter

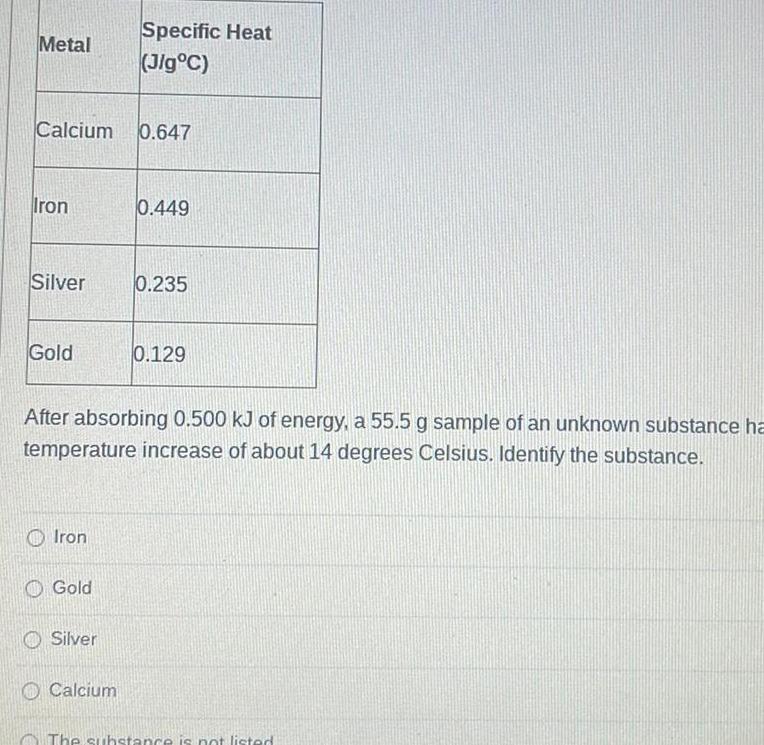
Physical Chemistry
GeneralMetal Calcium 0 647 Iron Silver Gold Iron Gold Specific Heat Jig C Silver After absorbing 0 500 kJ of energy a 55 5 g sample of an unknown substance ha temperature increase of about 14 degrees Celsius Identify the substance Calcium 0 449 0 235 0 129 The substance is not listed

Physical Chemistry
General4 What would a protein synthesized in the rough endoplasmic reticulum have to pass through to leave the cell What happens to the protein in this location

Physical Chemistry
GeneralWhich of the following is paramagnetic Select all that apply 1 N 2 F 3 0 21 4 N 5 0 2 000 1 05 04 N C K

Physical Chemistry
GeneralWhat is the element that is located in the 2nd Period and a Halogen Chlorine Nitrogen O Oxygen Flourine
Physical Chemistry
GeneralModel Factors Influencing Global Climate Change Long Term Cycles Consider the following elements as you construct your initial model The shape of Earth s orbit Explain how the earth s orbit givens us a warm climate and a cool climate 0 The tilt of Earth s axis Explain how the earth s axis givens us a warm climate and a cool climate Be sure to include labels to explain how both factors can lead to changes in global You will either tum in a hardcopy of your model or you will take a picture of it and paste it here


Physical Chemistry
GeneralThe endocrine system is a organs It uses hormones to energy level 1 network of glands and and your body s growth and development blood pressure fertility and injury stress and mood function and response to

Physical Chemistry
GeneralIn the following acid base equilibria of weak acids in water label the acid A the base B the conjugate acid CA and the conjugate base CB HClO aq H O 1 H O aq ClO aq H CO3 aq H O 1 H O aq HCO3 aq H O 1 CH3NH aq CH NH aq H O aq CH3COOH aq H O 1 CH3COO aq H O aq A Answer Bank CB CA B

Physical Chemistry
General0 548 moles of Ca NO3 2 was used to create a 2 6 M solution What is the volume of the solution

Physical Chemistry
GeneralPart D Mass to Mass Stoichiometry Calculate Give answers with 3 decimal places Date 16 Given this reaction 2 Al 3 Cl2 2 AICIs MMAICH3 133 33 g When 196 grams of aluminum combine with chlorine how many grams of AlCls are produced Period 17 Given this reaction 1 CuO 1 H 1 Cu 1 H 0 If 3 0 grams of hydrogen begin the above reaction how many grams of copper will be formed Part E Limiting and Excess Reactants Write the answer to each of the following questions on the line Given this reaction 1 Pb NO3 2 2 Nal 2 NaNO3 1 Pbl MM Pb NO3 2 331 208 15 0 grams of Pb NO3 2 and 15 0 grams of Nal are used to produce NaNO3 MM NaNO 84 994 18 How many grams of NaNO3 are produced 19 Which reactant was limiting 20 Which reactant was excess Part F Percent Yield Write the answer to each of the following questions on the line Given the reaction 1 Ba NO3 2 1 Na SO4 1 BaSO4 2 NaNO3 MM Ba NO3 2 261 335 A chemist began this experiment with 70 5 grams of Ba NO3 2 After mixing the reactants in solution an separating the products in the lab 42 45 grams of NaNO3 was obtained MM NaNO3 84 994 21 What was the actual yield of NaNO3 22 What is the theoretical yield of NaNO3

Physical Chemistry
General2 A galvanic cell is composed of these two half cells with the standard reduction potentials shown Co aq 2e o s E 0 25 vol 91 got you Cd aq 20 Cd s E 0 39 volt 2ulor grit What is the standard free energy change in kj for the cell reaction of this galvanic cell po y pp 8y

Physical Chemistry
GeneralWhich Inorganic Substance has a melting point of 101 C and a boiling point of 35 C Hydrogen Sulfide Hydrogen Chlorine Hydrogen Chloride

Physical Chemistry
General3 2 points Ripe strawberries produce enzymes called pectinases and cellulases These enzymes are catalysts in the process of breaking cell walls What is an enzyme Is enzyme assisted breaking of cell walls a chemical or a physical process

Physical Chemistry
Generalto have a specific heat capacity of 0 452 J g C Which substances have a higher specific heat capacity than Substance C Assume the masses of each substance are the same q mcAT Select 2 answers Change in Temperature vs Heat Added Temp C A C B Heat Energy Added kJ D

Physical Chemistry
GeneralAs the intermolecular attractive forces between molecules increase in magnitude do you expect each of the following to increase or decrease in magnitude Part A Part B Part C Part D Part E Part F Part G Critical temperature increase

Physical Chemistry
GeneralPart A Which assumptions are common to both kinetic molecular theory and the ideal gas equation 000 00 Repulsive forces among molecules are negligible The volume of gas molecules is negligible relative to the container volume Attractive forces among molecules are negligible The mass of gas molecules is negligible All gases have the same diffusion rate Molecules do not collide

Physical Chemistry
GeneralWhat is the difference between facilitated and simple diffusion across a cell membrane Wha types of chemicals travel by each method

Physical Chemistry
GeneralWhat is the formula for Boyle s law What is the relationship in Gay Lussac s law What law was demonstrated in the video



Physical Chemistry
GeneralWhich of the following is true for 0 50 M HNO3 strong O pH 3 5 O conjugate base concentrated

Physical Chemistry
General2 In one sentence describe the purpose of the following in this experiment a mashing the strawberries b using dish detergent c using table salt d using cold rubbing alcohol 4 poir

Physical Chemistry
General0 09 moles of Na SO4 are dissolved in 12 mL of solution What is the molarity of the solution

Physical Chemistry
Generalmolecules in the atmosphere can form en bonded dimers H O 2 The presence e dimers is thought to be important in the tion of ice crystals in the atmosphere and in mation of acid rain Part A What kind of intermolecular forces are involved in water dimer formation Submit Part B Request Answer The K for water dimer formation in the gas phase is 0 050 at 300 K and 0 020 at 350 K Is water dimer formation endothermic or exothermic endothermic exothermic

Physical Chemistry
GeneralLearning Goal To calculate the pH at the equivalence point for various types of titrations The equivalence point in an acid base titration is the point at which stoichiometrically equivalent quantities of acid and base have been mixed together At this point the reaction is complete because all analyte has been consumed by titrant On a titration curve the equivalence point is represented by the point of inflection where the curve changes concavity The figure Figure 1 shows the titration of 40 0 mL of 0 100 M HCl with 0 100 M NaOH When 40 0 mL of the NaOH solution is added the acid base neutralization reaction is complete When analyzing titrations involving weak acids or bases consider how the neutralization reaction will impact the Figure Hd 14 12 10 8 6 4 2 0 0 1 of 1 t Equivalence point 20 0 40 0 60 0 mL of 0 100 M NaOH added 80 0 Part B A 88 0 mL volume of 0 25 M HBr is titrated with 0 50 M KOH Calculate the pH after addition of 44 0 mL of KOH at 25 C Express the pH numerically View Available Hint s pH Submit Part C VAXO pH puzz VAXO Mang Consider the titration of 50 0 mL of 0 20 M NH3 Kb 1 8 x 10 5 with 0 20 M HNO3 Calculate the pH after addition of 50 0 mL of the titrant at 25 C Express the pH numerically View Available Hint s


Physical Chemistry
GeneralWhat type of intermolecular forces predominates between water and palmitic acid O dipole dipole attraction O London forces O hydrogen bonding Submit the larger carboxylic acid palmitic acid CH3 CH 14COOH O palmitic acid C We know that nonpolar molecules tend not to dissolve in water Does acetic acid contain a significant molecular fragment that is nonpolar acetic acid yes

Physical Chemistry
General3 Why didn t the amount of distilled water added have to be measured exactly

Physical Chemistry
GeneralDraw the Lewis structure for sulfite SO 2 with minimized formal charges Does this molecule exhibit resonance 0 of 2 points earned 3 attempts remaining opon ploctrons in H S and then draw the

Physical Chemistry
General3 Suppose sin 5 17 suppose is in Quadrant II and is in Quadrant IV Find the following sin 0 6 8 sin o Moreover

Physical Chemistry
GeneralK What is the molal concentration of the limiting reactant AFTER 37 complete reaction WRITE YOUR ANSWER IN SCIENTIFIC NOTATION WITH 4 SIGNIFICANT FIGURES ANY OTHER ANSWERS WILL BE MARKED WRONG You don t have to follow the Rules for SF operations Just round off your final answer so that you have a scientific notation with 4 SF 2 pc

Physical Chemistry
GeneralA solution was made by adding 800 g of ethanol C H5OH to 8 0 10 g of water What is the w w C H5OH A 9 1 B 10 C 91 D 99

Physical Chemistry
General9 If a chemist has 0 449 moles of C H60 how many moles of carbon will she get by decomposing the sample

Physical Chemistry
GeneralThe combustion of 35 0 g of O2 will produce of H O for the reaction below 4NH3 7024NO2 6H2O a

Physical Chemistry
GeneralWhich sample has the largest volume The density of aluminum is 2 7 g mL and the density of copper is 9 0 g mL A 1 0 g aluminum B 5 0 g aluminum C 1 0 g copper D 5 0 g copper

Physical Chemistry
General5 Pagos 594 601 Watch KCV 14 5 IWE plution is prepared by dissolving 29 0 g of cose C6H12O6 in 360 g of water The final ume of the solution is 384 mL Calculate the molarity of the solution Express your answer in moles per liter to three significant figures View Available Hint s M Submit Part B VAE VAE CANC Calculate the molality of the solution Express your answer in moles per kilogram to three significant figures View Available Hint s O mol L 1

Physical Chemistry
GeneralThe number of atoms in one mole oxygen gas is greater than equal to less than Previous than the number of atoms in one mole of helium gas

Physical Chemistry
General16 2 points In the reaction below how many grams of lithium oxide can be produced from 135 g of lithium phosphide 4 Li3P 3 02 6 Li O P4 117 g 78 g 312 g 465 g

Physical Chemistry
General21 To make 44 0 grams of carbon dioxide you must combine 12 0 grams of carbon with 32 0 grams of oxygen If a chemist combines 120 0 grams of carbon with 160 0 grams of oxygen how many grams of carbon dioxide will be made If a substance is leftover indicate whether it is carbon or oxygen and also determine how many grams are leftover

Physical Chemistry
General7 What is the mass of a CuCl2 sample if it contains 0 556 moles of the compound

Physical Chemistry
GeneralCalculate the solubility in g L of CaSO4 s in 0 250 M Na SO aq at 25 C The Ksp of CaSO4 is 4 93 x 10 5 solubility

Physical Chemistry
GeneralWhat classifies a substance as an Arrhenius base A The substance donates a proton B The substance accepts a proton C The substance increases the hydrogen ion concentration H in water D The substance increases the hydroxide ion concentration OH in water