General Questions and Answers

Physical Chemistry
GeneralType your answers in all of the blanks and submit
Consider the chemical reaction that occurs when 50.00 g of dinitrogen tetroxide (N₂O4, 92.02 g/mol) reacts with 47.50 g of
hydrazine (N₂H4, 32.06 g/mol) to produce nitrogen gas and water.
How many grams of excess reagent are left over after the reaction has run to completion if the percent yield of the reaction was
100%?
Excess reagent left over after the reaction with 100% yield =
reaction was only 75%?
12.804
Excess reagent left over after the reaction with 75% yield =
You are correct
Now consider how many grams of excess reagent are left over after the reaction has run to completion if the percent yield of the
9.618
You are incorrect
grams
X
grams

Physical Chemistry
GeneralIdentify the type of context clue used to identify the meaning of the underlined word.
Cindy detests, that is she dislikes intensely the thought of reciting her poem in front of
the entire school.
Antonym
Definition
Synonym

Physical Chemistry
GeneralSuppose there is Avogadro's number of mini marshmallows and they are used to cover the province of Manitoba, which has a
land area of 5.536 x 105 km². Each mini marshmallow has a diameter of 0.635 cm and a height of 2.54 cm. Assuming the
marshmallows are packed together so there is no space between them, to what height above the surface, in kilometers, will the
mini marshmallows extend?

Physical Chemistry
GeneralSuppose a sample of butane (C4H₁0) contains 193 g of carbon (C). Use unit analysis to calculate the mass of the butane sample.
Set up the unit analysis by dragging the necessary components into the conversion factor slots.
193 g Cx
X
E
X

Physical Chemistry
General7. Write the equilibrium constant expression for each of the following reactions: (3 pts))
4014
(a) 2TiCl3(s) + 2HCl(g)
2TICI4(s) + H₂(g)
SLA
(b) CH4 (g) + 2H₂S(g) CS₂ (g) + 4H2(g)
(c) Ag2C₂O4 (s) —2Ag* (aq) + C₂0₁² (aq)
youinel

Physical Chemistry
GeneralThe balanced chemical equation for the reaction is:
2+
3+
2+
8H* (aq) + 5Fe²+ (aq) + MnO (aq) 5Fe³+ (aq) + Mn²+ (aq) + 4H₂0 (1)
What kind of reaction is this?
- 1
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of Fe in the sample. Be sure your answer has 3
significant dinits
Oprecipitation
acid-base
redox
7
%

Physical Chemistry
GeneralExactly 10.0 gram of a solute was added to a graduated cylinder containing
80.00 mL of water. What is mass percent of the solute in this mixture to 3
significant figures?
11.1%
10.0%
11.0%
13.0%
12.5%

Physical Chemistry
GeneralAluminum reacts with oxygen to form aluminum oxide, Al₂O₂. Set up the calculation and solve for the mass ratio of aluminum
to oxygen in this compound.
mol Al x
mol O x
4
26.981 g/mol
Answer Bank
47.997 g/mol
5
53.962 g/mol
1
101.96 g/mol
3
= mass ratio
2
15.999 g/mol
mass ratio=

Physical Chemistry
GeneralAluminum reacts with oxygen to form aluminum oxide, Al₂O3. Set up the calculation and solve for the mass ratio of aluminum
to oxygen in this compound.
2
3
mol Al x
mol O x
26.981 g/mol
15.999 g/mol
= mass ratio
mass ratio=
1.62
Incorrect

Physical Chemistry
GeneralConsider the following reaction:
Fe + Br2 FeBr₂
If 53.2 grams of iron react with an excess of bromine gas, what mass of FeBr₂ can form?
(FeBr2 = 215.65 g/mol)
a) 13.8 g
b) 205 g
c) 226 g
d)
4.41 x 10-3
bo
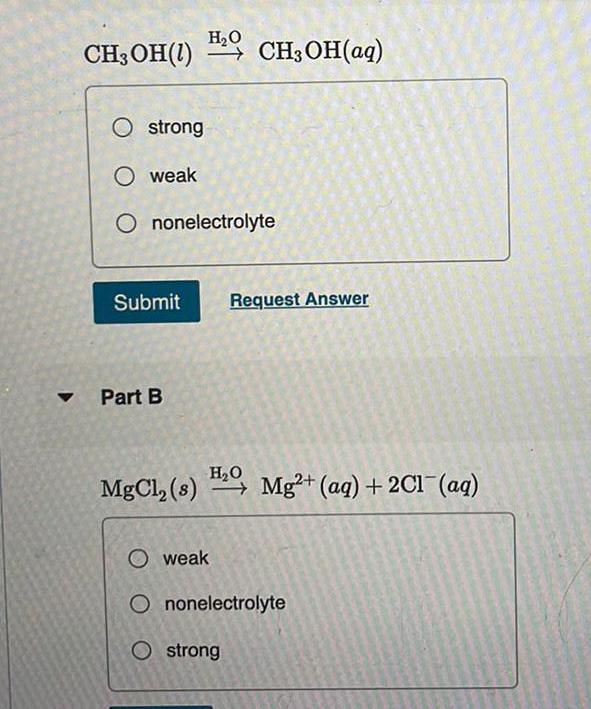
Physical Chemistry
General▼
H₂O
CH3OH(1) CH₂OH(aq)
O strong
O weak
Ononelectrolyte
Submit Request Answer
Part B
MgCl₂ (s) ₂, Mg2+ (aq) + 2Cl(aq)
O weak
Ononelectrolyte
Ostrong

Physical Chemistry
GeneralWrite the net ionic equation to show the formation
of a precipitate (insoluble salt) when the following
solutions are mixed. Write noreaction if there is
no precipitate.
▼
NaNO3(aq) and KBr(aq)
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no
precipitate is formed.
Submit
AΣO
A chemical reaction does not occur for this question.
Part C
Request Answer
?
K₂SO4 (aq) and Ca(NO3)2 (aq)
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no
precipitate is formed.
AΣO
Submit Request Answer
?
A chemical reaction does not occur for this question.

Physical Chemistry
GeneralIn this reaction, ZnBr₂ (aq) + K₂CO3 (aq) → 2 KBr (aq) + ZnCO3 (s)
all of the zinc (II) bromide is consumed and some potassium carbonate is left over. The ZnBr₂ is called the
a) product.
b) precipitate.
c) limiting reagent.
d) excess reagent.

Physical Chemistry
GeneralWrite the net ionic equation to show the formation of aprecipitate (insoluble salt) when the following solutions are mixed. Write noreaction if there is no precipitate.
AgF(aq) and KBr(aq)
Express your answer as a chemical equation. Identify all of the phases in your answer.
NaNO3(aq) and KBr(aq)
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no precipitate is formed.

Physical Chemistry
GeneralSuppose a sample of butane (C₂H₁) contains 193 g of carbon (C). Use unit analysis to calculate the mass of the butane sample.
up the unit analysis by dragging the necessary components into the conversion factor slots.
Set
193 g Cx
1 mol C₂H₁0
12.01 g C
1 mol C
X
58.12 g C₂H₁0
1 mol C₂H₁0
10 mol H
Answer Bank
4 mol C
4 mol C
X
10 mol H
1 mol C
12.01 g C
1.01 g H
58.12 g C₂H₁0

Physical Chemistry
GeneralConsider the reaction of copper(II) oxide with aluminum metal to produce copper metal and aluminum
oxide:
3 CuO(s) + 2 Al(s) → 3 Cu(s) + Al2O3(s)
-
If 1.69x1023 atoms Al react with an excess of CuO, what mass of Al2O3(s) is produced? Assume the
reaction goes to 100% completion.
Enter your response in decimal notation to three significant figures.

Physical Chemistry
GeneralWhy do the noble gases have basically no electron affinity?
They already have full valence shells of electrons, so they gain more electrons
They already have full valence shells of electrons, so they do not change energy if they gain more electrons
They already have full valence shells of electrons, so they lose electrons
They already have full valence shells of electrons, so they do not gain more electrons

Physical Chemistry
GeneralCalculate the reaction entropy (A S rxn) for the following reaction: 2 LIOH + CO2LI₂CO3 + H₂O
(A) -113.4 J/mol K
B) 200.6 J/mol K
C) 364 J/mol K
D) 313.8 J/mol K

Physical Chemistry
GeneralIn a certain reaction, Fe and O₂ combine to form iron(III) oxide.
33.5 moles of Fe and 23.2 moles of O₂ are placed in a container and the reaction proceeds with 100%
yield.
Note: Both answers must be correct for the question to be scored as correct
Which is the excess reactant?
O iron
oxygen
What is the amount of excess reactant left when the reaction reaches completion? Provide your answer in
decimal notation rounded to 1 decimal digit.
Enter Value
moles are left over.

Physical Chemistry
GeneralIdentify which of these molecules has the highest boiling point and give the reasoning why in terms of intermolecular force. H₂O, KCI, CO₂
KCI has the highest boiling point
Boiling point is in the order KCI>H2O>CO2
in KCI inter molecular force is ionic bond. That is formed by compleate tranfer of electrons. Hence break the bond is very difficult. Hence boiling point is higher. H2O has hydrogen bond hence its boiling point is higher than that of CO2)

Physical Chemistry
GeneralIdentify the spectator ions in the following molecular equation.
KBr(aq) + AgNO3(aq) → AgBr(s) +KNO3(aq)
Ag+ and Br
K+ and NO3
K+ and Br
Ag+ and NO3
There are no spectator ions in this reaction.


Physical Chemistry
GeneralCalculate the molecular weights for NH3 and SF6.
How many grams of \(\ce {NH_3}\) are needed to provide the same number of molecules as in \(0.45\) g of \(\ce{SF_6}\)?

Physical Chemistry
General6. True or False
A solution of a strong electrolyte will always have a higher conductivity than a solution of a weak electrolyte.
A solution of a strong electrolyte will always have a higher conductivity than a solution of a non-electrolyte.
A beaker contains a solution that has a low to medium conductivity reading. Is it appropriate to conclude that
the solution contains a weak electrolyte? Explain.

Physical Chemistry
GeneralChalk is a mixture of CaCO3 and CaSO4. When added to HCI, only the CaCO3 reacts according to the
reaction:
CaCO3(s) + 2 HCI (aq) → CaCl₂ (aq) + CO₂(g) + H₂(1)
When a 7.54 g piece of chalk is placed in HCI, 2.68 of CO2 is produced. What percentage of the chalk is
CaCO3?
41.2%
80.8%
61.9 %
59.1 %
37.3 %

Physical Chemistry
GeneralIdentify the literary device/figurative language from the following statement: "Out in
the porch's sagging floor, Leaves got up in a coil and hissed, Blindly struck at my knee
and missed."
Hyperbole
Metaphor
Similar

Physical Chemistry
GeneralBituminous coal is a type of coal that is composed of carbon with small amounts of hydrogen, oxygen, nitrogen, and sulfur. The
formula for a sample of bituminous coal is C₁37H97O,NS. Calculate the amount of carbon in 65.0 kg of the sample.
mass:
kg C

Physical Chemistry
GeneralCalculate the enthalpy change for the reaction:
P4O6(s) +2O₂(g) →→ P4O10(s)
given the following enthalpies of reaction:
P4(s) + 3O₂(g) → P4O6(s) ΔH = -1640.1 kJ
P4(s) + 5O₂(g) → P4O10(s) ΔH = -2940.1 kJ

Physical Chemistry
GeneralIf 12.3 g of aluminum carbonate (233.99 g/mol) is dropped into a solution that contains an excess
of sulfuric acid, what is the percent yield if 4.52 g of carbon dioxide is recovered?
3 H₂SO4(aq) + Al(CO₂)→ Al₂(SO)(aq) + 3 CO2(g) + 3 H₂O(l)

Physical Chemistry
General75.00 g of compound A (molar mass = 84.72 g/mol) and 13.17 g compound B (molar mass = 74.4 g/mol)
react completely to form 14.85 g compound C (molar mass = 41.89 g/mol) and 73.32 g compound D
(molar mass = 103.56 g/mol) with neither reactant in excess.
What is the coefficient for compound A in the chemical equation for this reaction when balanced in lowest
whole-numbers?

Physical Chemistry
General06 >
A patient is having an allergic reaction and takes a 12.5 mg tablet of the over-the-counter antihistamine diphenhydramine
hydrochloride, C17H22CINO. How many molecules of diphenhydramine hydrochloride are in this tablet?
molecules:

Physical Chemistry
GeneralAluminum metal can be recycled from scrap metal by melting the metal to evaporate impurities.
(a) Calculate the amount of heat needed to purify 1.00 mole of Al originally at 298 K by melting it. The
melting point of Al is 933 K. The molar heat capacity of Al is 24 J/(mol-K), and the heat of fusion
of Al is 10.7 kJ/mol.
(b) The equation for the overall process of extracting Al from Al-O, is shown below. Which requires less
energy, recycling existing Al or extracting Al from Al-O,? Justify your answer with a calculation.
Al₂0,(s) → 2 Al(s) + 0₂ (8) AH = 1675 kJ/mol,

Physical Chemistry
GeneralThe atom ratio in the compound nickel(II) phosphide has been found to be 0.667 atoms P
atom Ni
What is the mole ratio for this compound?
mole ratio:
Give the empirical chemical formula for nickel(II) phosphide by adding the necessary subscripts.
empirical formula:
NiP
What is the mass ratio of P to Ni in nickel(II) phosphide?
mass ratio:
mol P/mol Ni
g P/g Ni

Physical Chemistry
GeneralIdentify the literary device/figurative language used in the following lines from "A Red,
Red Rose" by Robert Burns.
"O my Luve's like the melodies,
That's sweetly play'd in tune."
O Hyperbole
Similar
O Metaphor

Physical Chemistry
GeneralNaNO3(aq) and KBr(aq)
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter noreaction if no
precipitate is formed.

Physical Chemistry
GeneralYou may want to reference (Page) Section 12.3
while completing this problem.
Determine whether a solid forms when solutions
confaining the following salts are mixed.
▼ Part G
▼
Enter the complete ionic equation when BaCl2 and KOH are mixed.
Express your answer as a complete ionic equation. Identify all of the phases in your answer. Enter noreaction if
no reaction occurs.
ionic equation:
A chemical reaction does not occur for this question.
Submit
AΣO
Part H
Request Answer
Review I Constants I Periodic Table
?
Enter the net ionic equation when BaCl2 and KOH are mixed.
Express your answer as a net ionic equation. Identify all of the phases in your answer. Enter noreaction if no
reaction occurs.

Physical Chemistry
GeneralDetermine the mass in milligrams of 0.402 mol NH CI.
mass:
Determine the mass in milligrams of 81.3 mmol H₂SO4.
mass:
mg NHẠCI
mg H₂SO4

Physical Chemistry
GeneralA scuba diver takes a 2.3 L balloon from the surface, where the pressure is 1.0 atm and the temperature is 35 °C, to a depth of 35 m, where the pressure is 4.5 atm and the temperature is 14 °C.
What is the volume of the balloon at this depth?
Express your answer in liters to two significant figures.

Physical Chemistry
GeneralWhat is the formula for ammonium sulfide? Capitalization and punctuation count.
ammonium sulfide
How many hydrogen atoms are in 2.00 mol of ammonium sulfide?
H atoms

Physical Chemistry
GeneralWrite a balanced half-reaction describing the reduction of gaseous dichlorine to aqueous chloride anions.
la
00
0
2.
X S ?

Physical Chemistry
GeneralGive the net ionic equation for the reaction (if any) that occurs when aqueous solutions of K2S and Fe(NO3)2 are mixed.
OK+ (aq) + NO3(aq) → KNO3(s)
Fe²+ (aq) + S2 (aq) + 2 K+ (aq) + 2 NO3(aq) → FeS(s) + 2 K+ (aq) + 2NO3(aq)
Fe²+ (aq) + S²-(aq) + 2 K+ (aq) + 2 NO3(aq) → Fe2+ (aq) + S2-(aq) + 2 KNO3(s)
Fe²+ (aq) + S2 (aq) → FeS(s)
-
No reaction occurs.

Physical Chemistry
GeneralPerform each pressure conversion.
24.9 psi to millimeters of mercury
Express your answer using three significant figures.
32.79 in. Hg to torr
Express your answer using four significant figures.

Physical Chemistry
GeneralChoose the correct answer.
The most common purpose of a thesaurus is
Oto avoid repeating the same word in your writing
to impress readers with your knowledge of synonyms
to impress readers with your knowledge of antonyms
All of the choices

Physical Chemistry
GeneralWhy do some people find it easier and quicker to use online dictionaries instead of
print dictionaries?
All of the choices
Online dictionaries contain less information than print dictionaries.
It can be easier and quicker to find words because you can just type them in instead o
searching for them in alphabetical order.
O Online dictionaries are written more simply and are easier to understand.
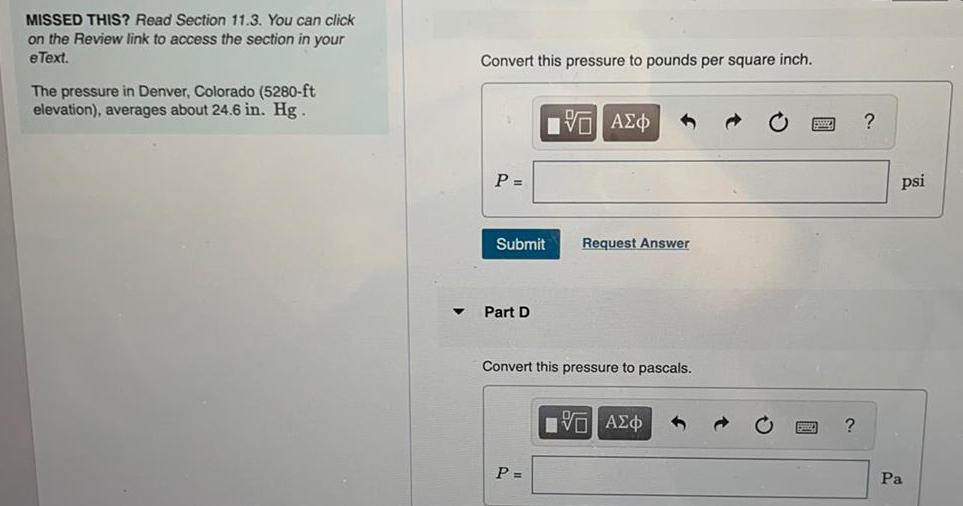
Physical Chemistry
GeneralMISSED THIS? Read Section 11.3. You can click
on the Review link to access the section in your
eText.
The pressure in Denver, Colorado (5280-ft
elevation), averages about 24.6 in. Hg.
Convert this pressure to pounds per square inch.
P =
Submit
Part D
D
P =
VAΣo
Convert this pressure to pascals.
Request Answer
D
VAZO
?
?
psi
Pa

Physical Chemistry
GeneralWhat are the 3 interactions that happens in a solution between the solute and the solvents? Describe the interactions in terms of salt dissolved in water.
1) Solute-solute interaction (x-x): They are the intermolecular force of attraction between Solute particles only. Hence Solute binds other solute molecules.
2) Solute - Solvents interaction(x-y): it is known as solvents. When salt(NaCl) is dissolved in water It dissociates in ion of Na+ and Cl- and they both are surrounded by water molecules due to this interaction. (Na+ by negative dipole of oxygen and Cl- by positive dipole of water
3) Solvents-Solvents interaction(y-y): They are intermolecular force of attraction between solvents particles only. Hence solvents binds other solvents molecules.
In case of salt and water Solute-Solvent forces are very much stronger. So they dissolves easily.

Physical Chemistry
GeneralThe following appears to be an entry from a
Narrow adjective
being of less than usual width <found a narrow opening in the fence that he was able
Synonyms fine, hairline, needlelike, paper-thin, skinny, slender, slim, slim-jim, thin,
ultrathin
Antonyms broad, fat, wide
dictionary
glossary
Othesaurus

Physical Chemistry
GeneralSulfurous acid (H₂SO3) is a polyprotic acid. Write balanced chemical equations for the sequence of reactions that sulfurous acid can undergo when it's dissolved in water.

Physical Chemistry
GeneralConsider the following balanced equation:
16HCl(aq) + 2KMnO4(aq) → 5Cl2(g) + 8H₂O(l) + 2KCI(s) + 2MnCl₂(aq)
If 35.4 moles of HCl(aq) and 7.63 moles of KMnO4(aq) are allowed to react to produce 13.4 moles of H₂O(l), what is the percent yield of the reaction?
Provide your answer in decimal notation, rounded to 1 decimal digit.

Physical Chemistry
GeneralIf ammonia can be produced from calcium oxide and ammonium chloride according to the following reaction. If 17.6 g of calcium oxide is used in the reaction and 7.43 g of ammonia is recovered, what is the % yield of the reaction?
CaO(s) + 2 NH4Cl(s)→ 2 NH3(g) + CaCl₂(s) + H₂O(g)