General Questions and Answers

Physical Chemistry
GeneralFor the following reaction, 4.04 grams of hydrogen gas are allowed to react with 28.1 grams of ethylene (C₂H4).
H₂ (g) + C₂H4 (9) C₂H6 (9)
What is the FORMULA for the limiting reagent?
What is the maximum amount of ethane (C₂H6) that can be formed?
What amount of the excess reagent remains after the reaction is complete?
![A vitamin A sample contains 2.0 x 102² atoms of carbon. What is the mass of the vitamin A sample?
[?] grams C20H30O](https://media.kunduz.com/media/sug-question/raw/72171813-1659274644.6632266.jpeg?w=256)
Physical Chemistry
GeneralA vitamin A sample contains 2.0 x 102² atoms of carbon. What is the mass of the vitamin A sample?
[?] grams C20H30O

Physical Chemistry
General1. The pH of an aqueous solution that is 2.7 x 10³ MHCI is What is the pOH? Circle one: ACID or BASE
2. The pH of an aqueous solution that contains 1.8 x 104 MHBr is What is the pOH? Circle one: ACID or BASE

Physical Chemistry
General2) Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen.
a) Write out the above equation in formula
b) balance the equation
c) Calculate the moles of hydrogen produced from 64.3 moles of hydrochloric acid
d) How many moles of zinc will produce 316 moles of zinc chloride?

Physical Chemistry
GeneralYou often hear the phrase, "You are what you eat." Which of the following statements is true?
The carbohydrates that you eat will cause your body to retain water, making you
look bloated.
The food we eat provides the fundamental material to keep our bodies
functioning.
Eating fats will make you fat because your body cannot digest fats.
If you eat a lot of protein, your hair and nails will grow faster.

Physical Chemistry
GeneralOne of the hydrates of FeCO3 is iron (II) carbonate monohydrate.
A 50.3 gram sample of FeCO3.H₂O was heated thoroughly in a porcelain crucible, until its weight remained constant. After heating, how many grams of the anhydrous compound remained?

Physical Chemistry
GeneralGaseous ammonia chemically reacts with oxygen (O₂) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of ammonia needed to produce 0.70 mol of nitrogen monoxide. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Physical Chemistry
GeneralA natural product has been isolated from the roots of a tropical shrub that was recently found on the Whatsamatta
University campus.
Sherman determined the chemical composition of the natural product to be 4.60% Nitrogen, 65.11% Carbon, 6.62% Hydrogen with the remaining percentage being Oxygen.
When RJ dissovled 1.00 grams of the natural product in 25.00 grams of camphor, the freezing point of the solution was lowered by 2.63 °C.
Useful information:
The kf for camphor; 40.0 °C kg/mol.
Density of the natural product-camphor solution: 1.20 g/ml
Vapor Pressure of pure solvent at 70.0 °C: 4.20 mm Hg
Molecular mass of camphor: 152.4 g/mol
Given this information, help RJ & Sherman answer the following questions.
a.Determine the molality of the solution. m
b.Determine the molecular mass of the unknown solute g/mol
c.Determine the molecular formula of unknown solute

Physical Chemistry
GeneralFor the positive ion, predict the formula of the simplest compound formed between each positive ion and the oxide ion. For the negative ion, predict the formula of the simplest compound formed between each negative ion and the aluminum ion. Name the compounds.

Physical Chemistry
GeneralWhat type of reaction is represented by the following equation:
Mg(CIO3)2(s) --> MgCl2(s) + 3 O2(g)
synthesis
combustion
single replacement
decomposition

Physical Chemistry
GeneralA. An element with the valence electron configuration 3s¹ would form a monatomic ion with a charge of _______
In order to form this ion, the element will ___ ____ electron(s) from/into the _____ subshell(s).
B. An element with the valence electron configuration 3s^23p^3 would form a monatomic ion with a charge of ______
In order to form this ion, the element will ___ ____ electron(s) from/into the _____ subshell(s).

Physical Chemistry
GeneralRank the following from lowest to highest in terms of their uncertainty in position? Assume that the uncertainty in velocity is the same for each of them.
1. a ethene molecule
2. a methane molecule
3. a methanol molecule
4. a methyl carbocation (CH3*)
a. 1<3<4<2
b. 1<3<2<4
C. 4<2<3<1
d. 4<2<1<3
e. 1<2<3<4

Physical Chemistry
GeneralThe nerve agent Tabun (GA) has the formula C H N O P. It is one of the lethal chemical compounds used in the chemical attack in the Kurdish city of Halajaba in 1988. How many carbon atoms are in 975 milligrams of Tabun? (Note: This amount of Tabun on the skin is potentially fatal.)

Physical Chemistry
GeneralSuppose you need 9.5 m of Grade 70 tow chain, which has a diameter of 3/8" and weighs 2.16 kg/m, to tow a car. How would you calculate the mass of this much chain?
Set the math up. But don't do any of it. Just leave your answer as a math expression.
Also, be sure your answer includes all the correct unit symbols.
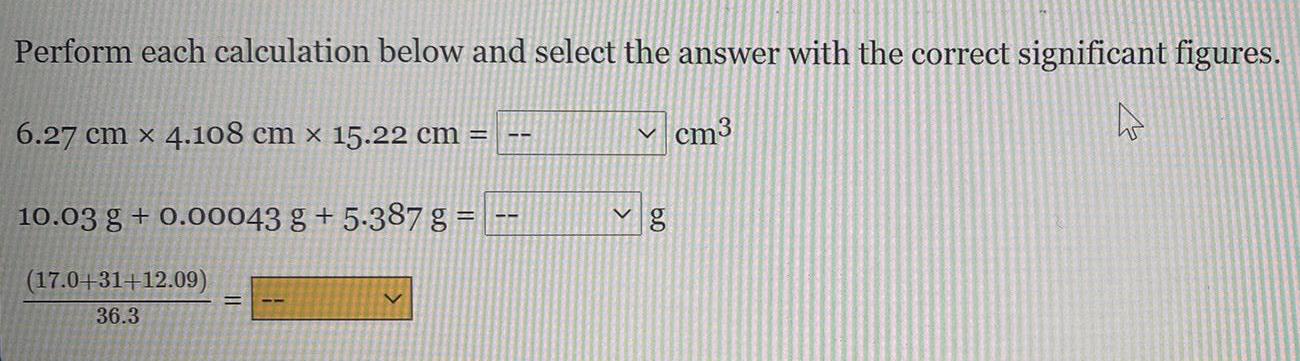
Physical Chemistry
GeneralPerform each calculation below and select the answer with the correct significant figures.

Physical Chemistry
GeneralThis is the chemical formula for methyl tert-butyl ether (the clean-fuel gasoline additive MTBE):
CH,OC (CH₂),
A chemical engineer has determined by measurements that there are 5.33 moles of carbon in a sample of methyl tert-butyl ether. How many moles of hydrogen
are in the sample?
Be sure your answer has the correct number of significant digits.

Physical Chemistry
GeneralBelow are 4 properties of gold. Which are physical properties? Check all that apply.
Pure gold is a vibrant yellow color, while fool's gold is darker yellow.
The density of gold is 19.3 g/mL, whereas the density of fool's gold is 5.02 g/mL.
Gold will not react with nitric acid, but most other metals will.
Gold is relatively soft and malleable, but fool's gold is brittle.

Physical Chemistry
GeneralUsing a graduated cylinder, you measure out 11 mL of water. You then go to an analytical balance and find the water to weigh 11.9273 g. What is the measured density of the water to the correct number of significant figures?

Physical Chemistry
GeneralPhosphoric acid, a mild acid used among other things as a rust inhibitor, food additive, and etching agent for cavity repair by dentists, can be made from elemental phosphorus in a two step process. In the first step, phosphorus and oxygen react to form diphosphorus pentoxide: In the second step, diphosphorus pentoxide and water react to form phosphoric acid (H,PO₁): P₂O(g) + 3H₂O(1) 2H, PO, (1) Suppose the yield of the first step is 88.% and the yield of the second step is 90.%. Calculate the mass of phosphorus required to make 1.0 kg of phosphoric acid. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.

Physical Chemistry
GeneralCarbon-14 and Nitrogen-14 both have the same mass number, yet they are different elements.
Which of the following statements is true?
Carbon-14 and Nitrogen-14 have the same number of neutrons.
Carbon-14 and Nitrogen-14 have the same number of protons.
Carbon-14 and Nitrogen-14 have different numbers of protons and neutrons.
Carbon-14 and Nitrogen-14 are isotopes.

Physical Chemistry
GeneralPart C
What is the charge associated with each side of the HCI molecule?
Express your answer in Coulombs to three significant figures.
What is the percent ionic character of the H-Cl bond?
Express your answer numerically to three significant figures.

Physical Chemistry
GeneralThe quantity of antimony in an ore can be determined by an oxidation-reduction titration with an oxidizing agent. The ore is dissolved in hot, concentrated acid and passed over a reducing agent so that all of the antimony is in the form of Sb³+ (aq). The Sb³+ (aq) is completely oxidized by an aqueous solution of BrO3(aq).
Complete and balance the equation for this reaction in acidic solution.
equation:

Physical Chemistry
GeneralA student reacts benzene, C6H6, with bromine, Br₂, to prepare bromobenzene, C6H5Br, and HBr.
C6H6Br2 --> C6H5Br + HBr
What is the theoretical yield of bromobenzene in this reaction when 28.2 g of benzene reacts with 60.1 g of bromine? grams
Which chemical is the limiting reactant? Select an answer
If the actual yield of bromobenzene was 20.8 g, what was the percent yield?
%

Physical Chemistry
GeneralChoose the sentence with the correct comma placement.
I took Adam, the one with the freckles to the movie last night.
I took Adam, the one with the freckles, to the movie last night.
I took Adam the one with the freckles, to the movie last night.

Physical Chemistry
GeneralKrypton, first discovered in samples of liquefied air, takes its name from the Greek word for
"hidden", kryptos. What is the experimental STP density of krypton if 3.70 g of the gas
occupies a 1013-mL flask at 25 °C and 1.00 atm? (Hint: Do not use the molar mass of
krypton!)

Physical Chemistry
GeneralChoose the sentence that is capitalized correctly.
I decided to take courses in Math and Statistics.
I decided to take courses in math and statistics.
I decided to take courses in math and Statistics.

Physical Chemistry
GeneralWhich definition best describes the term molar mass?
the mass, in ounces, of one mole of a substance
the mass in units of moles
the mass of one gram of a substance
the mass, in grams, of one mole of a substance

Physical Chemistry
GeneralWhich statement correctly describes an endothermic chemical reaction?
a. The products have higher potential energy than the reactants, and the ΔH is negative.
b. The products have higher potential energy than the reactants, and the ΔH is positive.
c. The products have lower potential energy than the reactants, and the ΔH is negative.
d. The products have lower potential energy than the reactants, and the ΔH is positive.

Physical Chemistry
GeneralIt has been shown experimentally that a beam of electrons can be diffracted. This is evidence that
electrons
are lighter than protons
have wave properties
have particle properties
are negative

Physical Chemistry
GeneralUsing only the letter names of the spaces of the treble clef staff, which word below could be
spelled?
Car
Feed
Cafe
Fade

Physical Chemistry
GeneralIdentify the correctly punctuated sentence below.
Jacob asked, "Why should I buy a bouquet?"
Jacob asked, "Why should I buy a bouquet"?
Jacob asked, Why should I buy a bouquet?
![The temperature is increased to 100° C:
Average Kinetic Energy [Select]
[Select]
increaes
decreases
remains the same
Average Velocity [Select]
Wall-Collision Frequency [Select]](https://media.kunduz.com/media/sug-question/raw/58763692-1659273242.764251.jpeg?w=256)
Physical Chemistry
GeneralThe temperature is increased to 100° C:
Average Kinetic Energy [Select]
[Select]
increaes
decreases
remains the same
Average Velocity [Select]
Wall-Collision Frequency [Select]

Physical Chemistry
GeneralChoose the option that is correctly hyphenated.
The accident in the show was a real letdown.
The accident in the show was a real let down.
The accident in the show was a real let-down.

Physical Chemistry
GeneralFor the following reactions, write the balanced molecular equation, the complete ionic equation and the net ionic equation. Make sure that the equations are balanced. You should write three equations.
a. Cs₂S(aq) + HgBr₂(aq) → CsBr(aq) + HgS(s)
b. Na₂CO₂(aq) + NH4Cl(aq) → NaCl(aq) + CO₂(g) + NH3(g) +H₂O(g)

Physical Chemistry
GeneralThe solubility in acetone of molecular compound M is measured and found to be 0.514 g/mL at 30. °C. Calculate the volume of a saturated solution of M in acetone that would contain 49.0 g of M at this temperature.
Be sure your answer has the correct unit symbol and 3 significant digits.

Physical Chemistry
GeneralChoose the sentence with the correct comma placement.
John, and I, have had our share of arguments.
John and I, have had our share of arguments.
John and I have had our share of arguments.

Physical Chemistry
GeneralA jet airplane reaches 801. km/h on a certain flight. How long does it take to cover 187. m?
Set the math up. But don't do any of it. Just leave your answer as a math expression.
Also, be sure your answer includes all the correct unit symbols.

Physical Chemistry
GeneralA powder contains FeSO4.7H₂O (molar mass= 278.01 g/mol), among other components. A 3.870 g sample of the powder was dissolved in HNO3 and heated to convert all iron to Fe³+. The addition of NH3 precipitated Fe₂O3.xH₂O, which was subsequently ignited to produce 0.554 g Fe₂O3.
What was the mass of FeSO4.7H₂O in the 3.870 g sample?
mass of FeSO4.7H₂O:

Physical Chemistry
General2. The quantity of Cl in a municipal water supply is determined by titrating the sample with Ag+. The precipitation reaction taking place during the titration is
Ag+ (aq) + Cl- (aq) -> AgCl(s)
(a) How many grams of chloride ion are in a sample of the water if 30.2 mL of 0.105 M Ag+ is needed to react with all the chloride in the sample?
(b) If the sample has a mass of 11.2.0 g, what percentage of Cl- does it contain?

Physical Chemistry
GeneralIf the theoretical yield of a reaction is 38.0
grams of product and the percent yield is 76%.
How many grams were actually produced?
29 g
37 g
31 g
2.0 g
49 8
27 g

Physical Chemistry
GeneralCombustion - Combustion reactions always have
(C,H,O,) combining with pure oxygen (O₂). The pr
"Incomplete Combustion" results from a lack
monoxide as a product instead of carbon dioxide.
based on the reactants.
1. ___CH, +____0₂ →
2. ___C₁0H₂2 + 0₂
3. _CH3OH + ___0₂ →
4. ___C12H2₂012 + ___0₂ →
5.CH3OC₂H5 +_0₂ →

Physical Chemistry
GeneralUse the chemical equation below to answer the following question.
SnO₂ + H₂ -> Sn + H₂O
The compounds to the left of the arrow are known as __________ in a chemical reaction.
A Chemicals
B Solutes
C Reactants
D Products

Physical Chemistry
GeneralA. Empirical Formula of Magnesium Oxide
mass of crucible and cover + magnesium metal
(before heating)
mass of crucible and cover
mass of magnesium metal
mass of crucible and cover + magnesium oxide
(after heating)
mass of combined oxygen
(after heating-before heating)
Show the calculation of the empirical formula for trial 1 (see Example Exercise 6.1).

Physical Chemistry
GeneralOn the state level, the executive branch is led by the____________.
Governor
Chief Justice of the State Supreme Court
President

Physical Chemistry
GeneralWhich of the following shows a specific audience in the thesis statement?
High school students should not lose an opportunity to learn something because they
are afraid to ask.
James Madison is one of those who wrote federal papers.
Photosynthesis is a process to convert light energy into chemical energy.

Physical Chemistry
GeneralHow does the author appeal to logos in the passage above?
By presenting specific numerical Information to support the argument
By stating that the study came from Harvard University
By using children to appeal to readers' emotions
By making a strong conclusion after presenting a research

Physical Chemistry
GeneralWhich best describes adaptation?
A. reproducing as a species
B. a short-term change in behavior in response to
a stimuli
C. inherited changes in response to environmental
factors
D. change in size as an organism ages

Physical Chemistry
GeneralRead the following lines taken from "The Silver Mine" written by Selma Lagerlof and answer the question that follows: "The King again stepped to the window. He apparently was in his best mood. All that was great and noble within him had been awakened. "He shall let the silver mine rest in peace. Since through all his life he has starved and worked to perfect a people such as these, he shall be permitted to keep them as they are. But if the kingdom is in danger'--"" ""The kingdom is better served with men than with money! When he had said these words, the King shook hands with the minister and stepped out of the study."
The excerpt can be categorized into which of the following options?
The theme of the narrative
The resolution reached by the king.
The hook of the story

Physical Chemistry
GeneralThe British government is a combination of __ and __
unitary and democratic forms
federal and democratic forms
dictatorship and unitary forms
Both unitary democratic and federal democratic forms

Physical Chemistry
GeneralDefine atonality.
Atonality is the absence of a key.
Atonality is the Inclusion of several keys of music.
Atonality is the use of only major chords.
Atonality is the use of only minor chords.