General Questions and Answers

Physical Chemistry
General7 5 0033 tand of the path 0034 WO0041 YDSE 42 In Young s experiment if the amplitude of interferring waves are unequal then the 1 contrast in the fringes decreases 2 contrast in the fringes increase 3 number of fringes will increase 4 number of fringes will decrease WO0042

Physical Chemistry
GeneralALLEN 43 Young s experiment proves that which of the 48 following fact 1 light is made up of particles 2 light is made up of waves 3 light is made up of neither waves nor particles 4 fringe width doesn t depend upon the spacing 49

Physical Chemistry
GeneralHT0258 241 The process in which the heat given to a system is completely transformed into work is for ideal gas 1 Isobaric process 3 Isothermal process 2 Isometric process 4 Adiabatic process am for isothermal and the relation between 20 HT0264

Physical Chemistry
General3 0 4 mm 4 0 2 mm WO0058 59 If a transparent medium of refractive index 1 5 and thickness t 2 5x10 m is inserted in front of the slits of Young s Double slit experiment how much will be the shift in the interference pattern The distance between the slits is 0 5 mm and that between slits and screen is 100 cm 2 2 5 cm 4 0 1 cm 1 5 cm 3 0 25 cm WO0059 64 1 0 2 3 0 4 A ver thicks light 1 blu 3 rec

Physical Chemistry
GeneralPre Medical Physics 101 hat which of the 48 In Young s double slit experiment wavelength of light is 6000A Then the phase difference between the light waves reaching the third bright fringe from the central fringe will be 1 zero 2 2 3 4x 4 6

Physical Chemistry
GeneralAcidified water is electrolyzed using an inert electrode The volume of gases liberated at STP is 0 168L The quantity of charge passed through the acidified water would be 1 96 500 C 2 9 650 C 3 965 C

Physical Chemistry
General247 300 calories of heat is supplied to raise the temperature of 50 gm of air from 20 C to 30 C without any change in its volume Change in energy per gram of air is 2 0 6 canternal 4 6 0 calories 1 zero 3 1 2 calories ession 251

Physical Chemistry
General1 Two coherent sources of different intensities send waves which interfere If the ratio of maximum and minimum intensity in the interference pattern is 25 then find ratio of intensities of sources 1 25 1 2 5 1 3 9 4 4 25 16 W00031 6 40 1 3 Sa ph

Physical Chemistry
General7 Two beams of light having intensities I and 41 interfer to produce a fringe pattern on a screen The phase difference between the beam is at point A and 2t at point B Then find out the difference between the resultant intensities at A and B 1 21 2 51 3 1 4 41 24 25

Physical Chemistry
GeneralPhysics A reversible refrigerator operates between a l temperature reservoir at T and a high temperatu reservoir at T Its coefficient of performance H given by

Physical Chemistry
General2 For the following redox reaction answer the following questions 4 pts CuS s NO Cu 0 SO NO g a What is the element that is oxidized b What is the element that is reduced c What is the oxidizing agent d What is the reducing agent 3 Balance the redox equation in 2 9 pts in acidic conditions Reduction half reaction Oxidation half reaction

Physical Chemistry
General1 R T T 3 R T T 2 R T T 4 R T T 225 When an ideal diatomic gas is heated at constant pressure the fraction of the heat energy supplied which increases the internal energy of the gas is 1 2 5 2 3 5 3 3 7 4 5 7 HT0248 Morcone

Physical Chemistry
GeneralD Two wave are represented by the equations y a sin oot and y a cos ext The first wave 1 Leads the second by t 2 Lags the second by 3 Leads the second by 4 Lags the second by KIN KIN

Physical Chemistry
Generalosjon Constant supplied gas is 05 7 IT0248 3 Heat is supplied to it 4 No change in pressure HT0253 231 Air is filled in a tube of the wheel of a car at 27 C and 2 atm pressure if the tube is suddenly bursts the final temperature of air will be y 1 5 21 251 1 33 C 3 21 6 C 2 0 C 4 240 C HT0254

Physical Chemistry
GeneralHT0259 237 An ideal gas is taken round the cycle ABCA In the 243 D cycle the amount of work done involved is W m 4P P 1 12 P V 3 3 P V 3V 2 4 P V 6 P V HT0260 2 3 c

Physical Chemistry
General15 Water is a polar solvent and carbon tetrachloride CCI is a nonpolar solvent Retinol vitamin A nonpolar and sucrose polar are dissolved in H O and CCL respectively Is this true or false Explain

Physical Chemistry
GeneralMetal oxides that dissolve in water are called Alkalis and Metal oxides that are soluble in water further dissolve to form Metal Hydroxides Differentiate between Alkalis and Metal Hydroxides regarding their solubility in w ater

Physical Chemistry
General34 A gas speciman in one vessel is expend isothermally to double its volume and a sim specimen in the second vessel is expand adiabatically the same extent then 1 In the second vessel both pressure and w done are more 2 In the second vessel pressure in more but work done isless 3 In the first vessel both pressure work d

Physical Chemistry
GeneralWO001 18 Amplitude of waves observed by two light source of same wave length are a and 2a and have a phas difference of n between them Then minimur intensity of light will be proportional to 1 0 2 5a 3 a 4 9a

Physical Chemistry
General7 Using the mass of copper you calculated above and the mass of malachite you used to make your sample determine the percent copper in your sample Enter this value here as a percentage

Physical Chemistry
General1 Ratio of intensity of two waves is 25 1 If interference occurs then ratio of maximum and minimum intensity should be 2 5 1 1 25 1 3 9 4 4 4 9 WO0021

Physical Chemistry
GeneralQ C CH CON When few drop of HC1 is added to aq solution of asprin its solubility will The sodium salt of aspirin Decreases O Increases O Remain same Q Asprin will decompose is an analgesic medicine is soluble in water while aspirin itself is not quite soluble 04

Physical Chemistry
General19 If the intensity of the waves observed by two coheren sources is I Then the intensity of resultant wave i constructive interference will be 1 21 3 1 2 41 4 None of the above WO001

Physical Chemistry
General3 PT T constant 4 p T constant HT0249 227 The value of internal energy in an adiabatic process 1 Remains unchanged 2 Only increases 3 Only diminishes 4 May diminish and may also increase HT0250

Physical Chemistry
General4 An ideal gas is carried through a thermodynamic cycle 1 P 10 bar en10 V 4 en10 litre P P P V P 1 bar V litre V V Consisting of two isobaric and two isothermal processes Calculate the net work in the entire in litre bar

Physical Chemistry
General1 7 AV V 3 y AV V 2 AV V 4 y AV V HT0239 217 An ideal gas at 27 C is compressed adiabatically to 8 27 of its original volume If y 5 3 then the rise in temperature is 1 450 K 2 375 K 3 675 K 4 405 K HT0240 3 0

Physical Chemistry
GeneralALLEN 222 Equal volumes of a perfect gas are compressed to half of their initial volumes The first is brought about by isothermal process and the second by adiabatic process then 1 Both temperature and pressure will increase in the isothermal process 228 2 In the isothermal process the temperature will decrease and pressure will increases 3 Both temperature and pressure will increase in 229 adiabatic process

Physical Chemistry
General0 If intensity of each of the two waves is I and they ar having phase difference of 120 when the wave are superimposed then the resultant intensity wi be 1 1 2 21 3 1 2 4 41 WO002

Physical Chemistry
GeneralTwo oxides of a metal contain 27 6 and 30 0 of oxygen respectively if the formula of the first oxide is M304 what will be the formula of the second oxide a M 03 c M304 ol b M403 d MO 0 27 6 M 72 4 Mail Me

Physical Chemistry
GeneralA 2 0 solution by weight of urea in water shows a boiling point elevation of 0 18 deg Molecular weight of urea 60 Calculate the latent heat of vaporization per gram of water 1 495 3 cal g 2 560 3 cal g 3 525 8 cal g 4 501 3 cal a

Physical Chemistry
General7 Four charges are placed at the circumference of the dial of a clock as shown in figure If the clock has only hour hand then the resultant force on a positive charge qo placed at the centre points in the direction which shows the time as 1 1 30 2 7 30 3 4 30 9 20208 12 q ES0017 Two small spheres each having a charge Q are

Physical Chemistry
Generald It is less reactive One mole of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of 27 C If the work done during the process is 3 kJ then final temperature of the gas is C 20 J K a 100K b 150K c 195K d 255 K oncts with NH on

Physical Chemistry
Generalsir can you plz tell me a method to compare electric cond uctivity of metals like which is better conductor among N a Ag Cu at room temperature Actually i thought Na is alkali metal and should be more r eactive than d block metals but answer is not Na Also from which chapter is this question basically from

Physical Chemistry
General4 Phenomenon of interference is not observed by two sodium lamps of same power It is because both waves have 1 not constant phase difference 2 zero phase difference 3 different intensity 4 different frequencies

Physical Chemistry
General224 One mole of an ideal gas at temperature T expends according to the law a constant The work done by the gas till temperature of gas becomes T is 1 3 R T T R T T 2 R T T 4 R T T 5 230 V to jon 20 231 A

Physical Chemistry
GeneralALLEN 218 For monoatomic gas the relation between pressure of a gas and temperature T is given by P T Then value of C will be For adiabatic process 5 1 3 2 25

Physical Chemistry
GeneralExplain why the addition of silver nitrate in the CoCI 2 solution generates an effect analogous to the equilibrium of iron III thiocyanate Fe H20 6 aq SCN a Fe SCN H2O s aq H2O Co H O 62 aq 4Cl aq CoCl4 aq 6H2O 1 Explain what is the effect of adding water at equilibrium position in the second reaction

Physical Chemistry
General02 The work by an ideal monoatomic gas along the cyclic path LMNOL is PA 3P 2P 1 PV 3 3 PV M 2V 2 2PV 4 4 PV HT0223 DE02 800 8A TARGET PHYENG MODULE 0301 THERMAL PHYSCS102

Physical Chemistry
General4 11 For distinct interference pattern to be observed necessary condition is that ratio of intensity of light emission by both the sources should be 1 2 1 2 1 2 n

Physical Chemistry
GeneralALLEN 203 For a gas C 4 96 cal mole K the increase in internal energy of 2 mole gas in heating from 340 K to 342 K will be 1 27 80 cal 2 19 84 cal CY Ar 209
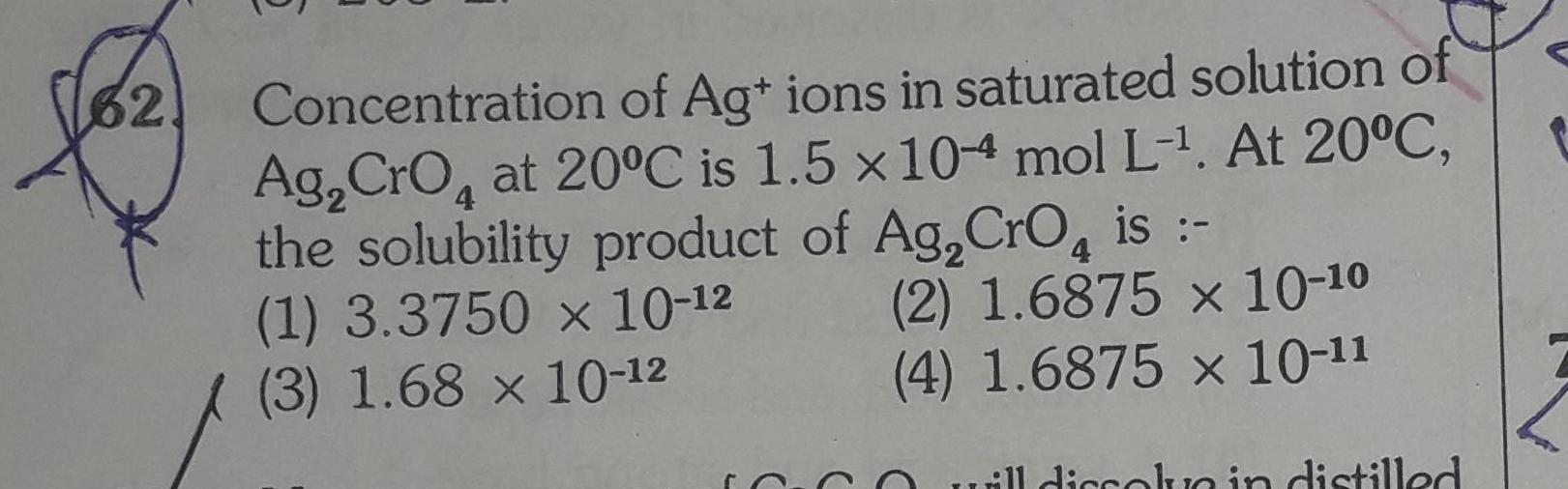
Physical Chemistry
General32 Concentration of Ag ions in saturated solution of Ag CrO at 20 C is 1 5 x 104 mol L At 20 C the solubility product of Ag CrO is 4 4 1 3 3750 x 10 12 3 1 68 x 10 12 2 1 6875 x 10 10 4 1 6875 x 10 11 till dissolve in distilled

Physical Chemistry
General12 A point charge q exerts a force F upon anothe point charge q If a third charge q be placed quit close to the charge q then the force that charg q exerts on the charge q will be 92 1 F 2 F 3 F 4 zero

Physical Chemistry
GeneralPre Medical Physics 93 CYCLIC ISOCHORIC ISOBARIC ISOTHERMAL ADIABATIC AND POLYTROPIC PROCESS 209 In the diagrams i to iv of variation of volume with changing pressure is shown A gas is taken along the path ABCDA The change in internal energy of the gas will be i E D A B ii iv 1 Positive in all cases i to iv D B B

Physical Chemistry
GeneralTwo point charges of 2 C and 6 C repel each other with a force of 12 N If each is given an additional charge of 4 C then force will become 1 4 N attractive 3 4 N repulsive 2 60 N attractive 4 12 N attractive

Physical Chemistry
Generalcyclic process shown on magnitude of the work done is P 202 P P 2 1 P V diagram the V V 2 2 3 P P V V 4 z P V P V HT0222 2 THERMAL PHYSICS EXBROSE P65

Physical Chemistry
General3 The work done by a gas taken through the closed process ABCA is SP P 2V 1 6P V 2 4PV B V 5V 3 P V 3 zero HT0229 ini con vol 1 2 3 4

Physical Chemistry
General94 Pre Medical Physics 212 Which of the following graphs correctly represents the variation of dV dP V with P for an ideal gas at constant temperature 1 3 2 4 B p

Physical Chemistry
Generalcharge 0002 ositive the net ES0006 Two point charges 9q and q are kept 16 cm apart Where should a third charge Q be placed between them so that the system remains in equilibrium 1 24 cur from 9q 3 24 cm from q 2 12 cm from 9q 4 12 cm from q

Physical Chemistry
Generalerature 199 1 kg of a gas does 20 kJ of work and receives Oxygen K E of 16 kJ of heat when it is expanded between two states A second kind of expansion can be found between the same initial and final state which requires a heat input of 9 kJ The work done by the gas in the second expansion is 1 32 kJ 2 5 kJ 3 4 kJ T0209 4 13 kJ HT0220

Physical Chemistry
General205 A system is taken along the paths A and B as shown If the amounts of heat given in these processes are Q and Q and change in internal energy are AU and AU respectively then 1 Q Q AU AU B 2 Q2 Qgi AU AUB 3 Q Q AU AU 4 Q Q AU AUB A V IT0336