Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
General organic chemistryWhich of the following is a FALSE statement?
a. Pressure is created by collisions between gas particles and the walls of container.
b. Atmospheric pressure is lower at higher altitudes.
C. A barometer is a device used to measure atmospheric pressure.
d. Standard temperature and pressure is 0 °C and 1 atm.
e. A small amount of the volume occupied by a gas is empty space.

Organic Chemistry
General organic chemistryWhat is the IUPAC name of this compound?
A) butanamide
B) N-methylbutanamide
C) N-dimethylbutanamide
D) N,N-dimethylbutanamide
E) dimethylbutanamide

Organic Chemistry
Practical DetectionA compound is found to contain 14.87% phosphorus and 85.13% chlorine by mass.
What is the empirical formula for this compound?

Organic Chemistry
General organic chemistryComplete the table below for calculating the molar mass of the compound sulfur dichloride.

Organic Chemistry
Practical DetectionShown below are the spectra (labeled A and B) for the reactant and product in a
bromination reaction. One spectrum shows C3H6Br2O and the other one shows
C3H6O. Identify which spectra is which, draw the two structures for the reactant and
product and then explain which peaks in the NMR spectra correspond to the
hydrogens in the structure.


Organic Chemistry
Chemistry in Daily LifeCopper (Cu) is a metal that is commonly used for electrical wiring. Determine both the number of moles of atoms and the number of atoms in a 1.20 g sample of copper.
How many Cu atoms are in the 0.0189 mol sample?


Organic Chemistry
General organic chemistryFind the oxidation numbers for each element in the following compound. Label positive values as "+X" and negative values as "-X" where X is the oxidation number you calculate.
Mg(MnO4)2

Organic Chemistry
Halogen DerivativesPredict the major product from the addition of HBr to the following molecules:
a) 3-methyl-1-pentene
b) 2,2-dimethyl-3-hexene

Organic Chemistry
General organic chemistryAt 473 K, the pressure of a sample of nitrogen is 1050 mmHg. What will the temperature be in
°C (assuming constant volume and amount of gas) if the pressure is decreased to 833 mmHg?
a. 102 °C
b. 324 °C-
C. 375 °C
d. 597 °C
e. 648 °C

Organic Chemistry
General organic chemistryYou have not correctly identified the
value of x. You know that x represents
the number of grams of sucrose. First,
calculate the number of moles of solute
in 250 mL of a 0.100 M solution. Then,
convert from moles to grams using the
molar mass of sucrose.

Organic Chemistry
PolymersIf pressure increases, which of the following variables would decrease? Assume all other
variables are held constant.
a. Temperature
b. Moles
c. Volume
d. Both Moles & Volume
e. Both Temperature & Volume

Organic Chemistry
Chemistry in Daily LifeConsider the following reaction:
2 Al +3 CuCl₂ →3 Cu + 2 AlCl3
Assuming 73.55 g of Al are reacted with 21.83 g of copper Il chloride, how much of the excess
reactant is left behind (unused)?
Identify the limiting reactant:
CuCl2
AI

Organic Chemistry
General organic chemistryWhen a 0.2481-g sample of titanium metal is reacted with oxygen, the final mass of the resulting compound is 0.4139 g. Determine the empirical formula of this titanium oxide.
HOW DO WE GET THERE?
What is the mass of each element present in the compound? (Enter your answers to four significant figures.)

Organic Chemistry
General organic chemistryHow many grams of Kr are there in a sample of Kr that contains 9.27×10^23 atoms?

Organic Chemistry
Chemistry in Daily LifeAn organic acid is composed of carbon (48.64%), hydrogen (8.18%), and oxygen (43.20%). Its molar mass is 74.08 g/mol. Determine the molecular formula of the compound.

Organic Chemistry
IsomerismAn alcohol is 62.04 % C and 10.43 % H by mass. The rest is oxygen. What is the empirical formula of the alcohol? Enter the elements in the order C, H, and then O.

Organic Chemistry
General organic chemistryVinegar contains carbon, hydrogen, and oxygen with percent masses of 40.01% C, 6.70% H, and 53.29% O, respectively. Determine the empirical formula of the compound.
HOW DO WE GET THERE?
What is the mass of each element in 100.0 g of vinegar? (Enter your answers to two decimal places.)

Organic Chemistry
General organic chemistryAn organic acid is composed of carbon (62.04%), hydrogen (10.43%), and oxygen (27.55%). Its molar mass is 116.16 g/mol. Determine the molecular formula of the compound.

Organic Chemistry
General organic chemistryHow many grams of Si are there in a sample of Si that contains the same number of moles as a 107 gram sample of Co?

Organic Chemistry
Chemistry in Daily LifeHow many ATOMS of nitrogen are present in 3.94 grams of dinitrogen tetroxide ?
How many GRAMS of oxygen are present in 4.10×10²2 molecules of dinitrogen tetroxide ?

Organic Chemistry
PolymersA sample of Sc weighs 96.7 grams. Will a sample of Be that contains the same number of atoms weigh more or less than 96.7 grams?
Calculate the mass of a sample of Be that contains the same number of atoms.

Organic Chemistry
Aldehydes & KetonesSynthesize the following compounds from the given starting material. You can add on
on any alkyl/ alkyl halide under 8 carbons or any necessary inorganic reagent needed (this
includes triphenyl phosphine (Ph3P). Please draw all intermediates and reagents
necessary to get to the product.

Organic Chemistry
Practical DetectionDuring formation of photochemical smog, methane react with ozone to form an aldehyde. How many moles of methane will be oxidized by 4 mole of ozone?

Organic Chemistry
Chemistry in Daily LifeWhich of the following types of map would best show the three
dimensions of Earth's surface?
road map
topographic map
weather map
tectonic map

Organic Chemistry
General organic chemistryA compound is found to contain 55.39 % boron, 8.280 % hydrogen, and 36.33 % chlorine by mass.
What is the empirical formula for this compound?

Organic Chemistry
General organic chemistryOzone (O3) is formed in the earth's upper atmosphere by the action of solar radiation on oxygen
molecules (O2). Write a balanced equation for the formation of ozone from oxygen.
Balance the following equations:
a. Ca(OH)2+HCl-CaCl2+H2O
b. Al +O2-Al2O3
C. CH3CH3+02-CO2+H2O
d. AgNO3+MgCl2-AgCl +Mg(NO3)2
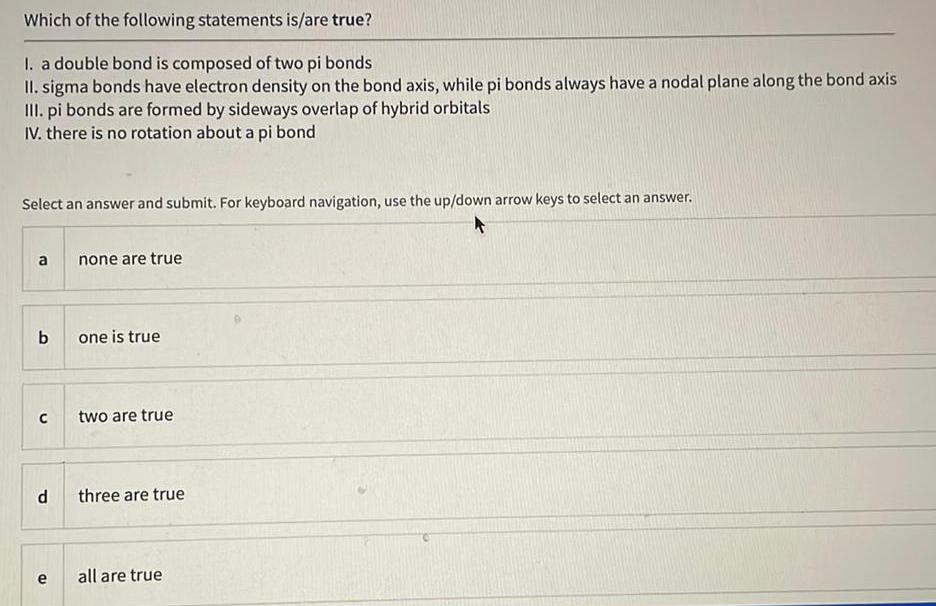
Organic Chemistry
General organic chemistryWhich of the following statements is/are true?
I. a double bond is composed of two pi bonds
II. sigma bonds have electron density on the bond axis, while pi bonds always have a nodal plane along the bond axis
III. pi bonds are formed by sideways overlap of hybrid orbitals
IV. there is no rotation about a pi bond
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a none are true
b one is true
c two are true
d three are true
e all are true

Organic Chemistry
Practical DetectionFind the element phosphorus, P, on the periodic table.
a. What is the average atomic mass of phosphorus?
b. What is its atomic number?
c. Predict which isotope you would find in greatest abundance for phosphorus.

Organic Chemistry
General organic chemistryA compound 'P' containing Bromine in it, on treatment with
NH3 produces 'Q', which is solid at room temperature and is
free from Bromine atom. The solid Q contains 49.3% Carbon,
9.59% Hydrogen, 19.18% Nitrogen and 21.93% Oxygen. 'Q' on
reaction with Br₂ and Ca(OH)₂ gives a product 'R'. The
product 'R' can give carbylamine test and give ethene on
reaction with excess of CH3 - I followed by heating with
moist Ag₂O.

Organic Chemistry
Practical DetectionHow many ATOMS of sulfur are present in 2.76 grams of sulfur dichloride ?
How many GRAMS of chlorine are present in 9.76x1022 molecules of sulfur dichloride?

Organic Chemistry
IsomerismA solution of two diastereomers
contains 77.0% of (3S,4S)-tert-
butyl-3-amino-4-
hydroxypyrrolidine-1-carboxylate
and 23.0% of (3S,4R)-tert-butyl-3-
amino-4-hydroxypyrrolidine-1-
carboxylate. Calculate the percent
diastereomeric excess (%e.e.) of
the (3S,4S)-tert-butyl-3-amino-4-
hydroxypyrrolidine-1-carboxylate in
this solution.

Organic Chemistry
General organic chemistryA compound is found to contain 37.47 % carbon, 12.61 % hydrogen, and 49.92 % oxygen by mass.
To answer the question, enter the elements in the order presented above.
QUESTION 1:
The empirical formula for this compound is
QUESTION 2:
The molar mass for this compound is 32.05 g/mol.
The molecular formula for this compound is

Organic Chemistry
IsomerismA solution of a chiral compound was
prepared by dissolving 15.67 g in
16.00 mL of solvent. The solution
was placed in a tube that is 1.00 dm
long.
What is the specific rotation of light
if the observed rotation was
determined experimentally to be
+62.41°?

Organic Chemistry
IsomerismA compound having an approximate molar mass of 210.0-215.0 g has the following percentage composition by mass:
carbon, 33.80 %
hydrogen, 1.428 %
nitrogen, 19.72 %
oxygen, 45.07 %
Determine the empirical and molecular formulas of the compound.
(Enter the elements in the order: C, H, N, O.)
Empirical formula:
Molecular formula:

Organic Chemistry
General organic chemistryA compound was analyzed and was found to contain the following percentages of the elements by mass: sulfur, 94.09%; hydrogen, 5.91%. Determine the empirical formula of the compound.
Empirical formula:

Organic Chemistry
General organic chemistryA compound having an approximate molar mass of 165.0-170.0 g has the following percentage composition by mass:
carbon, 42.86 %
hydrogen, 3.598 %
nitrogen, 25.00 %
oxygen, 28.54 %
Determine the empirical and molecular formulas of the compound.
(Enter the elements in the order: C, H, N, O.)
Empirical formula:
Molecular formula:

Organic Chemistry
HydrocarbonsUse the References to access important values needed for this question.
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below.
Molecular Formula.
Empirical Formula
C = black
H = gray

Organic Chemistry
HydrocarbonsUsing the average atomic masses for each of the following elements, calculate the number of atoms present in each of the following samples.
a. 80.16 amu of calcium
atom(s)
b. 1471.2 amu of tungsten
atom(s)
c. 164.82 amu of manganese
atom(s)

Organic Chemistry
General organic chemistryA compound is found to contain 26.73 % phosphorus, 12.09 % nitrogen', and 61.18 % chlorine by mass.
To answer the question, enter the elements in the order presented above.
QUESTION 1:
The empirical formula for this compound is____.
QUESTION 2:
The molar mass for this compound is 115.9 g/mol.
The molecular formula for this compound is_____.

Organic Chemistry
General organic chemistryOne mole of H₂O and one mole of CO are taken in a 10 litre vessel and heated to 725 K. At equilibrium, 40 percent of water (by mass) reacts with carbon monoxide according to the equation:
H₂O(g) + CO(g) ⇒ H2(g) + CO2(g)
Calculate the equilibrium constant for the reaction.

Organic Chemistry
Alcohols and PhenolsCalculate the percentage composition for SiO2.
Mass percentage of silicon = ____%
Mass percentage of oxygen =____%


Organic Chemistry
General organic chemistryA 5.746 gram sample of chromium is heated in the presence of excess fluorine. A metal fluoride is formed with a mass of 12.04 g. Determine the empirical formula of the metal fluoride
Enter the elements in the order Cr, F
empirical formula =
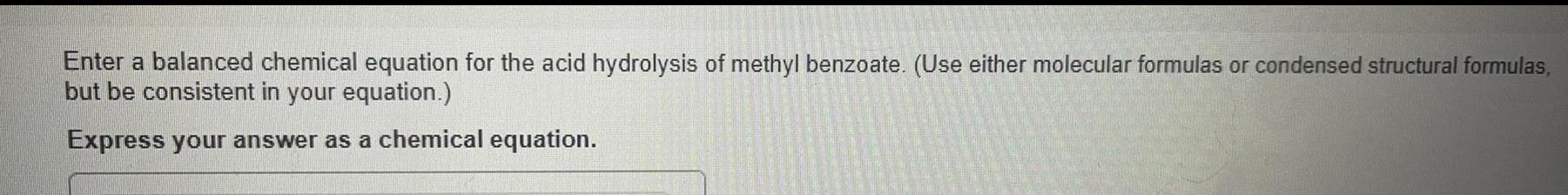
Organic Chemistry
General organic chemistryEnter a balanced chemical equation for the acid hydrolysis of methyl benzoate. (Use either molecular formulas or condensed structural formulas,but be consistent in your equation.) Express your answer as a chemical equation.

Organic Chemistry
Practical DetectionA compound is found to contain 26.96 % sulfur, 13.45% oxygen, and 59.59 % chlorine by mass. What is the empirical formula for this compound?
To answer the question, enter the elements in the order presented above.

Organic Chemistry
General organic chemistryA 42.96 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed with a mass of 78.03 g. Determine the empirical formula of the metal sulfide.

Organic Chemistry
General organic chemistryCalculate the percentage composition for CBr4.
Mass percentage of carbon =
Mass percentage of bromine =

Organic Chemistry
Practical DetectionA compound having an approximate molar mass of 253.0-258.0 g has the following percentage composition by mass:
Determine the empirical and molecular formulas of the compound.