General Questions and Answers

Physical Chemistry
General2 12 3 6 4 15 147 What is the electronic configuration of an element in its first excited state which is isoelectronic with O 1 Ne 3s 3p 3d 2 Ne 3s 3p 3 Ne 3s 3p 3d 4 Ne 3s 3p5 148 The quantum number of 20th electron of FeZ 26 would

Physical Chemistry
General8 40 of rotating molecules in higher ste is given by the No e No e ja Nye selar 0061696 20200 020064696 2020064696 202

Physical Chemistry
Generalc No change 33 If the maximum velocity and acceleration of a particle executing SHM are equal in magnitude the time period will be a 1 57 c 6 28 b Decreases d None b 3 17 d 12 56

Physical Chemistry
General6 A particle executing a simple harmonic motion has a period of 6 sec The time taken by the particle to move from the man position to half the amplitude starting from the mean position is a 3 A W N W S C S b d S 1 S

Physical Chemistry
GeneralD Which of the following statements are correct for SO gas a It acts as bleaching agent in moist conditions b It has linear geometry c It s dilute solution is used as lubricant d It can be prepared by the reaction of dilute H SO with metal sulphide

Physical Chemistry
Generaltwo simple of cot T 6 0 35 m 9 A particle performing SHM has time period path length 4 cm The displacement from mean position at which acceleration is equal to velocity is a Zero b 0 5 cm c 1 cm d 1 5 cm 2r and Oscillat

Physical Chemistry
General14 Photons of frequency 3 2 x 1016 Hz is used to irradiate a metal surface the maximum kinetic energy of the emitted photo electron is 3 of the energy of the 4 irradiating photon What is the threshold frequency of the metal a 2 4 x 1025 Hz c 1 6 x 10 5 Hz 5 The total number of orbitals in the principal shell of Het b 2 4 x 1016 Hz d 8 x 1015 Hz

Physical Chemistry
General5 A river water sample assume density 1 00g mL S 0 28 g kg contains the following Ion Ca2 Mg2 Nat K HCO3 SO42 CH Concentration mg kg Calculate the molarity of each ion 42 10 18 2 132 50 15

Physical Chemistry
General147 What is the electronic configuration of an element in its first excited state which is isoelectronic with O 2 Ne 3s 3p4 1 Ne 3s 3p 3d 4 Ne 3s 3p5 3 Ne 3s 3p 3d 148 The quantum number of 20th electron of r

Physical Chemistry
GeneralIdentify the statement which is correct Question Type Single Correct Type 1 2 3 Milk is an example of homogeneous solution Adsorption process is entropy driven process In case of CsCl crystal 8 Cs ions occupy next nearest neighbour position to each Cs ion Aqueous solution of ethylene 4 glycol can be used as

Physical Chemistry
Generala 1 5 c 7 8 b 3 5 d 12 7 73 Sulphuryl chloride SO Cl reacts with H O to give a mixture of H SO HCl Aqueous solution of 1 mole SO Cl will be neutralised by a 4 moles of NaOH b 2 moles of Ca OH d None of these c Both a b
Physical Chemistry
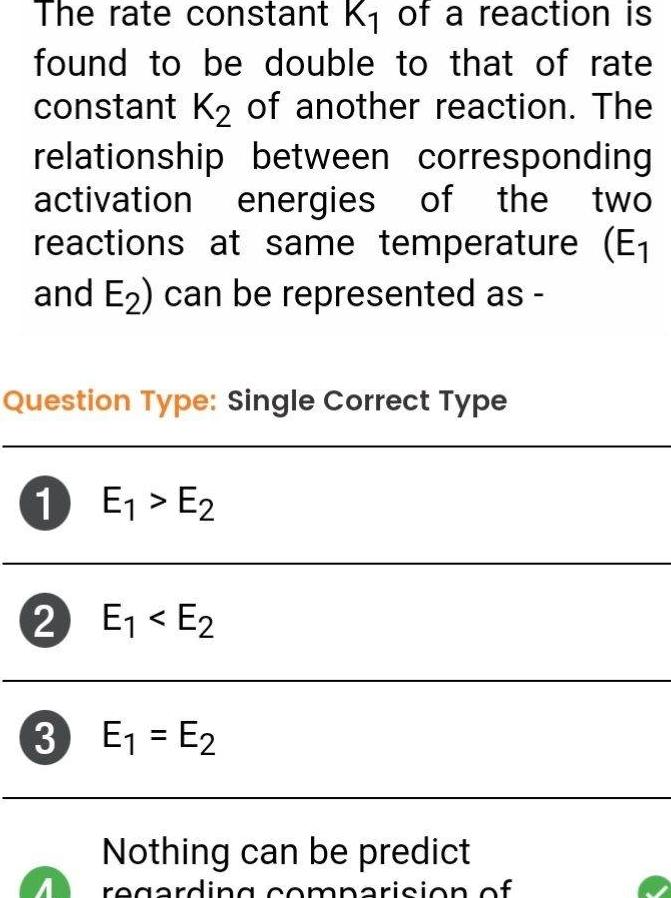
GeneralThe rate constant K of a reaction is found to be double to that of rate constant K of another reaction The relationship between corresponding activation energies of the reactions at same temperature E and E can be represented as two Question Type Single Correct Type 1 E E 2 E E 3 E E Nothing can be predict regarding comparision of

Physical Chemistry
GeneralWhat type of reaction is aluminium hydroxid e to aluminium oxide A Thermal dissociation reaction b Electrolytic decomposition reaction C Thermal decomposition reaction involvi ng a change in colour

Physical Chemistry
General56 Under isothermal condition a gas at 300 K expands from 1 L to 4 L against a constant external pressure go of 2 atm The heat absorbed by the gas is Aut 3 40 1 2 200 J Avtw 4 600 J n 1 L atm 100J 1 100 J 3 300 J

Physical Chemistry
GeneralCalculate the lattice energy of LiF s given that A AH for Lithium is 155 2 kJ mol sub B C D E A of Fluorine 333 kJ mol E AH for LiF s 594 1 kJ mol a 1011 6 kJ mol c 906 6 kJ mol AHis of 1 2 mole of F g is 75 3 kJ diss I E of Lithium 520 kJ mol b 2056 6 kJ mol d 573 6 kJ mol

Physical Chemistry
GeneralMoment of force can be defined as the product of force and distance from the line of action of force to the moment Centre Answer A O maximum B O any C

Physical Chemistry
General0 Cortisone is a molecular substance containing 21 atoms of carbon per molecule The mass percentage of carbon in cortisone is 69 98 What is the molar mass of cortisone A 176 5 B 252 2 D 360 1 C 287 6

Physical Chemistry
GeneralAt a certain temperature the solubility of the salt AB is S moles per liter The general expression for solubility product will be 1 K x y S 2 K xy 3 K x y S 4 K x y Say

Physical Chemistry
GeneralThe density of a pure substance is its mass per unit volume The density of ethylene glycol has been measured to be 1097 Calculate the mass of 455 mL of ethylene glycol Be sure your answer has a unit symbol and the correct number of significant digits Solution O O O O O P m m V m m V 455 1 097 b0 m 414 77 g 415 g g L Write down the definition of density Solve for mass Put in the data given Use the calculator Round to the correct number of significant digits

Physical Chemistry
General8 Schottky defect in crystals IS 1 Density of the crystal is increased 2 Unequal number of cations and anions are missing from the lattice 3 An ion leaves its normal site and occupies an interstitial site 4 Equal number of cations and anions are missing from the lattice

Physical Chemistry
GeneralWhen 100 mL of 1 0 M HCI was mixed with 100 mL of 1 0 M NaOH in an insulated beaker at constant pressure a temperature increase of 5 7 C was measured for the beaker and its contents Expt 1 Because the enthalpy of neutralization of a strong acid with a strong base is a constant 57 0 kJ mol this experiment could be used to measure the calorimeter constant In a second experiment Expt 2 100 mL of 2 0 M acetic acid Ka 2 0 x 105 was mixed with 100 mL of 1 0 M NaOH under identica conditions to Expt 1 where a temperature rise of 5 6 C was measured Consider heat capacity of all solutions as 4 2 J g K and density of all solutions as 1 0 g mL 1 2015 3 Enthalpy of dissociation in kJ mol of acetic acid obtained from the Expt 2 is A 1 0 B 10 0 C 24 5 D 51 4 The pH of the solution after Expt 2 is A 2 8 C 5 0 B 4 7 D 7 0 your A Dr Sup 20 whiny om bo state

Physical Chemistry
Generalc Both a b d None of these 74 A sample of pure compound contains 1 15 g of sodium 3 01 1022 atoms of carbon and 0 1 mol of oxygen atom Its empirical formula is a Na CO3 b NaCO c Na CO d Na CO 2

Physical Chemistry
GeneralHow many electrons are present in the kernel of P Z 15 1 2 3 Correct Answer 1 POLON 3 2532 10

Physical Chemistry
GeneralA sample of 10 gm activated charcoal was brought into contact with CO gas contained in a vessel of 1 2 litre capacity at 27 C The pressure of CO was found to fall from 760 torr to 570 torr What volume of CO gas in mL is adsorbed per gm of charcoal at 570 torr and 27 C Assuming negligible volume of charcoal Correct answer 40 00

Physical Chemistry
GeneralWhich of the following is the strongest base 1 CH NH pK 9 42 3 CH N CH pK 8 94 2 CH NHCH pK 9 15 4 CH NHCH pK 8 89 ession

Physical Chemistry
General1 A current liberates 0 504 g of hydrogen in 2 hours the amount of copper liberated from a solution of CuSO by the same current flowing for the same time would be 1 31 8 g 3 15 9 g CI 2 63 6 g 4 6 36 g 77 Na Corr 2 3

Physical Chemistry
GeneralSir for me balancing equation by ion electron method is more simpler as compared to the other method so can I gi ve more importance to this is it important to study the ot her Is there any questions that we can only get the answer b y doing this oxidation number method

Physical Chemistry
GeneralAns 2 70 Compound PdC1 6H O is a hydrated complex 1 molal aqueous solution of it has freezing point 269 28 K Assuming 100 ionization of complex calculate the molecular formula of the complex K for water 1 86 K kg mol 1 Pd H O CI 2 Pd H O C1 C1 2H O 3 Pd H O C1 C1 3H O 4 Pd H O CI 1 4H O 4 C CH Br Br Ar 74 1 2

Physical Chemistry
General19 4 I A ns np5 B n 1 d 0 ns C n 1 d5 ns D n 1 d 0 ns np6 1 A q B s C p D r 2 A R C 4 II P 9 R r for s n 4 2 A s B q C p D r AY

Physical Chemistry
GeneralWhich one is a wrong statement 2018 a Total orbital angular momentum of electron in s orbital is equal to zero b An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers c The value of m for d is zero d The electronic configuration of N atom is 1s 2s 2s 2p 2p 2p 1 1 114

Physical Chemistry
General3 Ag s 4 HBr aq 644 in the given rex the HCHO 1 2 Ag NH aq 3OH aq 2Ag s reducing HCOO aq 4NH aq 2H O 1 1 HCHO 1 3 HCOO aq 2 Ag NH3 aq 4 NH aq

Physical Chemistry
GeneralIf S S S and S are the solubilities of AgCl in water 0 01 M CaCl 0 01 M NaCl and 0 5 M AgNO solutions respectively then which of the following is true 2 S S S S 4 S S S S 1 S S S S 3 S S S S

Physical Chemistry
General26 What will be the output of the following C code include int main int i 23 char c 23 if i c printf Yes n else printf No n Yes Depends on the compiler Depends on the standard

Physical Chemistry
GeneralAccording to third law of thermodynamics the entropy at 0 K is zero for a Elements in their stable form b Perfectly crystalline solids c Substance as 1 atm and 25 C d N 0

Physical Chemistry
GeneralThe heat of neutralisation of four acids A B C and D when neutralised against a common base are 13 7 9 4 11 2 and 12 4 kCal respectively The weakest among these acids is a A c C b B d D

Physical Chemistry
General2 15 9 g 4 63 5 g A quantity of electric charge that brings about the deposition of 4 5 g Al from Al at the cathode will at produce the following volume STP of H g from H at the cathode 3 11 2 L 1 44 8 L 2 22 4 L 4 5 6 L

Physical Chemistry
GeneralWhat happens when sodium is added to water A gas is evolved II The temperature of the water increases III A clear colourless solution is formed A I and II only B I and III only C II and III only D I II and III 1

Physical Chemistry
GeneralIn which of the following chemical equations the abbreviations represent the correct states of the reactants and products involved at reaction temperature a 2H2 g 02 g 2H2O 1 b 2H2 g 02 1 2H2O 1 c 2H2 1 02 1 2H2O g d 2H2 g 02 g 2H2O g 8 41 PM

Physical Chemistry
GeneralThe wavelength in A of an emission line obtained for Li during a electronic transition from n 2 to n 1 is R Rydberg constant a 3R 4 b 27R 4 c 4 3R d 4 27R

Physical Chemistry
GeneralGurukripa an example of redox Rexn following rexn is not CAREER INSTITUTE 29 34 1 H PO aq 4AgNO3 aq 2H O 1 H PO4 aq 4Ag s 4HNO3 aq 2 N H 1 2H O 1 N g 4H O 1 3 2 K s F g 2KF s 4 BaCl aq H SQ g 35

Physical Chemistry
General6 1 16 If 0 224 L of H gas is formed at the cathode the volume of O gas formed at the anode under identical conditions is 1 0 224 L 2 0 448 L COMMERCIAL VOLTAIC CELLS 3 0 112 L 4 1 12 L

Physical Chemistry
General16 The solubility of AgCN in a buffer solution of pH 3 is x The value of x is Assume No cyano complex is formed K AgCN 2 2 10 16 and K HCN 6 2 10 10 sp 2 1 9 x 10 5 4 1 6 x 10 6 1 0 625 x 10 6 3 2 2 x 10 16

Physical Chemistry
Generalc CH3COOH 4 7 33 Given Enthalpy of ionization of two acids AH HCN 45 2 kJ mol AH CH3COOH 2 1 kJ mol Which relationship for the two acids is true a pK HCN pK CH3COOH pK HCN PK CH3COOH 1 What is the hydronium ion concentrat d CH3CH COOH 4 88 b pK HCN PK CH3COOH CE 45 2 d pK HCN 2 1 PK CH3COOH

Physical Chemistry
GeneralThe photograph shows silica packets which are used to absorb moisture in the packaging of certain products How did the silica minerals in these packets form y DO NA ROSA AWE GEL OA By the chemical precipitation of sedimentary rock O B By the weathering of sediment OD By the crystallization of magma C By the melting of metamorphic rock

Physical Chemistry
GeneralTwo acid HX and HY have strength ratio 5 2 their heat of neutralization are in the ratio of 5 4 If 40 gm of NaOH is added to a mixture of acids containing total 1 equivalent of HX and HY The total heat evolved is Q What is the ratio of Q and Q2 where Q is heat of neutralization of HY 1

Physical Chemistry
General3 The Kps solubility product for CuCl silver salt is 1 9 0 4 x 10 7 and the molar solubility is given by Solubility S Kps 1 2 1 9 0 4 x 10 7 1 2 Solubility S 4 4 x 10 4 M What is the uncertainty in solubility for CuCl

Physical Chemistry
GeneralA certain current liberates 0 5 g of hydrogen in 2 hr How many grams of copper can be deposited by the same current flowing for the same time in a copper sulphate solution 1 12 7 g 2 15 9 g 3 31 8 g 4 63 5 g

Physical Chemistry
General0 The threshold wavelength for ejection of electrons from a metal is 330 nm The work function for the photoelectric emission from the metal is h 6 6 1034 J s A 1 2 x 10 18 J C 1 2 x 10 12 J B 6 0 10 19 J D 6 0 10 12 J

Physical Chemistry
General11 A bubble of gas released at the bottom of a lake increases to eight times its original volume when it reaches the surface Assuming that atmospheric pressure is equivalent to the pressure exerted by a column of water 10 m height the depth of the lake is a 80 m b 90 m c 40 m e 70 m d 10 m Korala DMTY

Physical Chemistry
Generalare fuf vedox rex 3 I which of the 4 F following Rexn 3 After IIII III III 1 I CuO s H g Cu s H O g II Fe O3 s 3CO g 2Fe s 3CO g III 4BCl g 3 LiAH s 2B H g 3LiCl s 3AICI s IV 4NH g 50 g 4NO g 6H O g 1 2 3 4 344