General Questions and Answers

Physical Chemistry
General4 15 3 10 4 15 49 A gas expands from 3 dm to 5 dm against a constant 49 3 atm 3 dm 5 dm de pressure of 3 atm The work done during the expansion is used to heat 10 mol of water at temperature 290 K Find Yuraka f hell 290 the final temperature of water if the specific heat of water 4 18 Jg K 1 298 808 k 3 292 808 k 2 290 808 k 4 300 k 5 5 1 faf3 4 181 K 1 298 808 k 3 292 808 k Sy 3 M 2 290 808 k 4 300 k 4 Volume g H gas ST Hence work 1 22 4 L a 2 5 6 L at Idm33 11 2 L 11 4 44 8 L Given N g The sta

Physical Chemistry
GeneralThe product of following reaction will be Br OH Br Br Br H 0 Br Br 8 8 OH Product Br OH Br OH OH Br OH An electric discharge is passed through a mixture 77 78 79 1 For WH K 3 Wh pho 1 3 Ho are Cor
Physical Chemistry
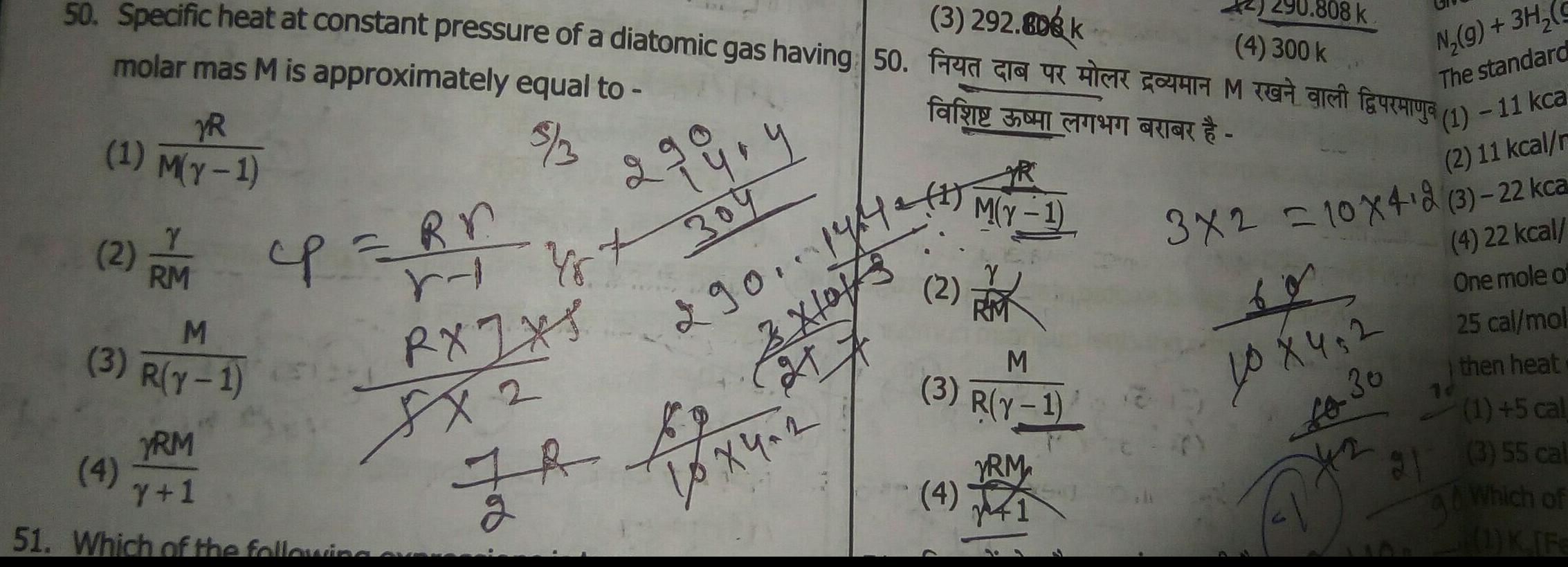
General3 292 808 k 4 300 k 50 Specific heat at constant pressure of a diatomic gas having 50 FK COM O faucurya molar mas M is approximately equal to far CRICK 3 3 YR 1 M y 1 Y 2 2M cp Rr RM M 3 R Y 1 RA 8x2 YRM 4 Y 1 51 Which of the following 2 99 4 304 290 1942 Xlo r Yst JR By 27 2 10x4 2 2 M Y 1 M 3 R Y 1 YRM 4 41 808 k N g 3H g The standard 1 11 kca 2 11 kcal r 3x2 10x4 2 3 22 kca 4 22 kcal One mole of 25 cal mol then heat 1 5 cal 31 3 55 cal Which of 1 K Fe 40 X 4 2 Lo 30 A

Physical Chemistry
GeneralZero Marks 0 In all other cases Assume isotope of chlorine present on the unknown planet are 34C1 and 38 Cl If average molecular weight of Cl is found to be 35 What is the sum of moles of proton and neutron in 7 gm of sample of chlorine

Physical Chemistry
GeneralOne mole of an ideal gas is allowed to expand freely and adiabatically into vacuum until its volume has doubled A statement which is not true concerning this expression is 1 a c AH 0 b AS 0 AE 0 d W 0

Physical Chemistry
General5 24 256M NITE NA D 0 089 Vapour density of a gas if its density is 0 178 g L at NTP is A 0 178 B 2 C 4 In an organic compound of molar mass greater than 100 containing only C H and N the percentage of

Physical Chemistry
GeneralA 2 00g serving of uni contains 7 2 ug essential nutrient compound if the compound is 22 2177 nitrogen by mass and one molecule of this compound contains 7 nitrogen atoms which is the molar mass of this nutrient in g mol

Physical Chemistry
GeneralWhat is the enthalpy change in kJ of a chemical reaction that raises the temperature of 250 0 ml of solution having a density of 1 25 g mL by 3 33 OC The specific heat of the solution is 3 74 J g K Select one a 6 51 b 7 43 c 8 20 d 3 89 X e 3 89

Physical Chemistry
General22 0 078 gms of a gaseous hydrocarbon occupy 44 8 ml volume at 1 atm and 273 C The empirical formula of the hydrocarbon is CH Find total number of atoms in one the molecule of the hydrocarbon Chenen STI

Physical Chemistry
General63 For the process H O atmosphere pressure the correct choice is 1 AS 0 and AS 0 system 0 0 3 AS w BSE H O g at T 100 C and 1 63 H O l H O g 100 C fouT 4 AS system system surroundings 2 AS 0 and AS system 0 and AS 0 and AS DSPARTY surroundings 0 4 A sample of liquid in a thermally insulated container 64 3 surroundings DS fly surroundings DSURG 2120 1 AS 0 and AS Fight 2 AS Fach 3 AS Fac 0 and AS 0 Ra Ra 0 and AS 4 AS 0 and AS fa 0 0 0 1

Physical Chemistry
Generalrule Dau s ty of nt of is m m 73 For an atom or ion having single electron compare the energies of the following orbitals S a spherical symmetrical orbital having two spherical nodes S an orbital which is double dumb bell and has no radial node S an orbital with orbital angular momentum zero and three radial nodes S an orbital having one angular and one radial node 1 S S S S 3 S S S S 2 S S S S 4 S S S S

Physical Chemistry
General4 1 1 g sample of copper ore is dissolved and Cu aq is treated with KI The I so liberated required 12 12 mL of 0 1 M Na S O3 solution for titration What is of Cu in the ore

Physical Chemistry
GeneralNegative Marks 1 If wrong option is selected The degree of dissociation of A is 10 then find molecular weight of A for given reaction when vapour density of mixture is 60 A g B g C g O 120 O132 O 60 O 40

Physical Chemistry
GeneralDuring a polytropic process 10lbs of an ideal gas whose R 40 ft lb lb R and Cp 0 25 BTU lb R changes state from 20psia and 40 F to 120psia and 340 F Determine I b AU C AS d Q e f Pdv Vdp

Physical Chemistry
General62 The following sequence of reactions may be 66 A sam used to extract zinc from its sulphide 10 the many Atomic weight of Zn 65 S 321 1 52 2ZnS 30 2ZnO 250 3 5 ZnO C Zn CO 30 mi 90 mL How many tons of Zn can be obtained from 32 33 tons of ZnS assuming that the yield is 75 for each reaction 1 5 3 12 2 2 100 4 16 4 67 produ formu 1 C 68 The S 31

Physical Chemistry
General16 An open vessel at 27 C is heated until two fifth of the air assumed as an ideal gas in it has escaped from the vessel Assuming that the volume of the vessel remains constant the temperature at which the vessel has been heated is a 750 K c 750 C b 500 K d 500 C

Physical Chemistry
GeneralSodium and oxygen combine to form two c ompounds one being Na20 The amount of sodium in the other compound is 59 by we ight Find the formula of this compound

Physical Chemistry
General3 3 5 3 6 4 5 6 5 5 Which of the following represents the unit of vanderwaal constant a 1 L mol 1 3 L atm mol 2 atm L mol 2 4 L atm mol 2 79 Under identical conditions solvolysis of which 78

Physical Chemistry
General1120 Average weight 11 2 100 Average atomic weight of an element M is 51 7 If two isotopes of M are 50M and 52M them calculate the percentage of occurrence of 50M in nature 50M X 52M X 100 x 100 x average atomic weight W X1 W X2 LI 51 7 50xx 52xx

Physical Chemistry
GeneralThe incorrect information s is are a The conductivity of a soap solution decreases sharply at CMC b Tyndall effect is more effective in gold sol in comparison to the rubber sol c The elevation in boiling point of an alcoholic solution of sulphur is less than that of its sol in water if mass of sulphur present per unit volume of mixture is same in both cases d CMC value of CH CH NH Cl will be less than that of CH CH 6 COONa

Physical Chemistry
GeneralNumber of spectral lines of Balmer series obtained when electrons are de excited from th shell to ground state in sample of H atoms is 1 n 1 3 n 3 2 n 2 4 n 4

Physical Chemistry
General71 72 3 35 4 45 The ratio of anion radius to cation radius of a crystal is 10 9 3 Then the coordination number of the cation in the crystal is 1 2 2 4 3 6 4 8 Which lot gorract

Physical Chemistry
GeneralWhich of the following salts is responsible for temporary hardness of water CaCl2 Ca NO3 2 Ca HCO3 2

Physical Chemistry
GeneralC Complete the sentences by choosing the correct abstract noun from the box difference respect belief freedom 1 2 3 childhood patience 4 5 6 hunger wisdom Children must be allowed to enjoy their must be valued Rakesh believes that he will be an astronaut one day It is this in himself that will help him succeed for Ms Brown I have great When I am hungry I get very irritable I cannot bear Our elders are wise because of their experience We must respect their 7 Do not be impatient always pays in the end 8 Weather and climate are two different things Let us understand the between them Fountable and Uncountable Nouns

Physical Chemistry
General31 One mole of one of the sodium salts listed below having carbon content close to 14 3 produces 1 mole of carbon dioxide upon heating atomic mass Na 23 H 1 C 12 0 16 The salt is a C H COONa b NaHCO3 c HCOONa d CH COONa

Physical Chemistry
GeneralA Column I Element B Compound C Mixture D H O E Fe s S s in powdered form P Q R S T U Column II Can t be decomposed into simpler substances by physical methods Can be decomposed into simpler substances by chemical methods Can t be decomposed into simpler substances by chemical methods Uniform composition Consists of one type of atoms Consists of 2 or more type of atoms

Physical Chemistry
GeneralNX is produced by the following step of reactions M X MX 3MX2 X M3X8 M3X8 N CO3 NX CO2 M304 How much M metal is consumed to produces 206 gm of NX Take at Wt of M 56 N 23 X 80 A 42 gm C 14 3 gm B 56 gm D 7 4 gm

Physical Chemistry
GeneralNitromethane Fuel having a chemical formula CH3O2N is burned in an engine at a fuel air equivalence ratio of 0 88 and a temperature of 990 K The molar fraction of CO2 is Select one a 0 472 b 0 518 c 0 247 d 0 158 e 0 089

Physical Chemistry
GeneralWhich of the following is not disproportionation reaction 1 P NaOH NaH PO PH 2 BaC N Ba CN 3 Hg 1 Hgl Hg 4 Cl 20H CIO The ratio of macres of Cl H O alament A attached

Physical Chemistry
Generals over solvent pressure has ative aspect of solubility of a gas with pressure has been ble effect on the solutions of solids and liquids The terms of Henry s Law y s Law Sunt of gas dissolved w per unit volume of solvent is proportional to its pressure PA i e XA A or XA KHPA where units of k is bar or atm e above result is sometimes also written as PA or PA KH XA XA Slope k atm gas ts pressure har inits of k is atm or bar pressure of the gas over the solution onality constant known as Henry s law constant w constant is different for each gas solvent dissolved per unit mass of solvent e of Cas K i Temp Ku k bar Application of Henry s Law a In the production of carbonated beverages Why cold drink water bottles are sealed under high pressure According to Henry s law solubility of gas increases with increase in pressure of the solution that is why cold drink bottles are sealed under high pressure so that more CO can be dissolved in it b In the deep sea diving For those people who do deep sea diving nitrogen and oxygen get dissolved in their blood under the effect of pressure of water This dissolved oxygen gets utilized in the metabolic activities but nitrogen remains as such When a person comes out of the sea this nitrogen which was dissolved in blood comes out in the form of bubbles which is very painful This is called de compression sickness c Combination of hemoglobin and oxygen in lungs Lungs oxygen Hb Oxy Hb Tissues Partial pressure is low When air enters lungs partial pressure of the oxygen is high This oxygen combines with hemoglobin to form oxyhemo globin but the tissues has less partial pressure of oxygen Thus in tissues oxyhaemoglobin breaks into oxygen and this released oxygen is used in metabolic activities ILLUSTRATION 8 7 If N gas is bubbled through water at 293 K how many millimoles of N gas would dissolve in 1 liter of water Assume that N exerts a partial pressure of 0 987 bar Given that Henry s law constant for N at 293 K is 76 84 kbar Solution The solubility of gas is related to its mole fraction in the aqueous solution The mole fraction of the in the solution gas uloted by applying Henry s law Thus

Physical Chemistry
General3 5 33 10 4 None of these 30 mL of a gaseous hydrocarbon requires 90 mL of oxygen for complete combustion and produces 60 mL of CO then the molecular formula of hydrocarbon is 1 C H 2 C H 3 C H 4 C H

Physical Chemistry
Generalappropriate number of significant figures Solution Radius of sphere r 1 141cm Volume of sphere Express its volume to an 1111 4 significant figures x 3 14 1 41 cm 11 736cm 3 11 72cm into 4 significant figures

Physical Chemistry
Generalare they two type of acceleration when they absorb electromagnetic radiation they highly acclerated and when they donot absorb electromagnetic radiation they are not highly acclerated but its own acclerated for which there is no chance it s stability 15 48

Physical Chemistry
General68 Which of the following molecule has longest C C bond 68 r length 1 CH C CH 2 CH CH CH CH 3 3 CH3 C CH CH 1 CH3 4 CH3 C CH

Physical Chemistry
General49 Which of the following resonating structure contributes 49 A SERIE ERCAN BAIE BE equally to the resonance hybrid 1 CHg C CH CH CH3 CH3 CH3 C CH CH CH3 CH3 2 CHO C NH CHI CINH O 3 CH C 00 CH C 00 O MAAR 1 CH3 C CH CH CH CH CH CH3 C CH CH CH3 CH3 2 CHg C NH2 CHI C NH O 00 3 CH C tan Batch PCBZ 2 C 8 00 CH C 00 0

Physical Chemistry
General180 A vessel contains 14 g 7 moles of hydrogen and 96 93 180 14 g 7 moles of oxygen at STR Chemical reaction is induced by passing electric spark in the vessel to produce water till one of the gases is consumed completely The temperature is brought back to it s starting value 273 K The pressure in the vessel is 1 0 1 atm 2 0 2 atm 3 0 3 atm 4 0 4 atm 0 lm Spark STP gr f ER EMI ga 1 0 1 atm 2 0 2 atm 3 0 3 atm 4 0 4 atm 273Kata FORT PVETRT at O 10x25

Physical Chemistry
General105 The standard electrode potential of Zn Zn is 0 76 V and that of Ca2 Cu is 0 34 V The emf V and the free energy change kJ mol respectively for a Daniel cell will be a 0 42 and 81 b 1 1 and 213 c 1 1 and 213 d 0 42 and 81

Physical Chemistry
GeneralOn dissolution of NH Cl in water pH of water decreases It is due to hydrolysis of H 1 NH ion 2 CH ion 3 Both NH and CH ion 4 No hydrolysis takes place NHuel M 0 to NH NM CINECL Hitech H

Physical Chemistry
GeneralIn the following reaction Cu H O 3 OH Al H O Cu H O AI H O OH A B C D A A is an acid and B the base B A is a base and B the acid C C is the conjugate acid of A and D is the conjugate base of B D C is conjugate base of A and D is the conjugate acid of B

Physical Chemistry
GeneralA Match the statements to the correct question tags 1 Hamida can t whisper 2 3 They hadn t been polite She looked very happy I shouldn t have gone there The sun shone brightly He was rude I am present It hasn t been corrected 4 5 6 7 8 9 You aren t feeling ill 10 They haven t been informed a wasn t he b have they c are you d aren t l e didn t it f can she g should I h has it i didn t she j had they

Physical Chemistry
GeneralHCHO Carbon monoxide on reaction with H in the presence of cobalt catalyst gives CH3OH hr CH3COOH m

Physical Chemistry
General56 If the angle of incidence of X ray of wavelength 3 which produces a second order diffracted beam from the 100 planes in a simple cubic lattice with interlayer spacing a 6 is 30 the angle of incidence that produces a first order diffracted beam from the 200 planes is a 15 b 45 c 30 d 60

Physical Chemistry
GeneralWhich of the following statements is are correct A At low temperatures hydrogen can be adsorbed more than deuterium on metal surface B Activation energy of deuterium is more than hydrogen in its reaction with halogen C Both H H and D D bond lengths are same D Heat of fusion of deuterium is more that of hydrogen

Physical Chemistry
GeneralC Haemoglobin blood and gold sol are negative charge colloids D Casein act as emulsifier for milk Given H 0 KMnO H SO MnSO H O O K SO which is correct statement A Number of gm equivalent of H O and KMnO reacts in the ratio of 5 2 B Number of moles of MnSO and O produced in the ratio of 5 2 C Equivalent mass of H SO is 29 4 D 100 ml 2 27 V H O reacts completely with 160 ml 0 5M KMnO in given reactic Space for Rough Work

Physical Chemistry
GeneralThe percentage of Fe in 3 oxidation state in Fe0 9 O is A 78 B 90 C 10 D 22 If law of conservation of mass to hold true then 20 8 g of BaCl on reaction with 9 8 g of

Physical Chemistry
GeneralFor the reaction CO g O g CO g AH 67650 cal 2 at 25 C Calculate AH at 100 C given that the required molar heat capacities are as follows Cp CO g 6 97 cal C 1 Cp CO2 g 8 97 cal C 1 Cp 02 g 7 00 cal C 1 O 54 6 cal O 67650 4 cal O 67684 4 cal 67762 5 cal

Physical Chemistry
GeneralArrange pH of the given compounds is decreasing order 29 feptera anfory 01 b ufera che G hs econ d 3 a Phenol c Formic acid 1 a b c d 2 b a d c 3 c b d a 4 d c a b b Ethyl alcohol d Benzoic acid a f c hitties and Coo H 1 a b c d 2 badc 3 c b d a A d h Batch PCBZ 1 to 3

Physical Chemistry
General50 How many grams of a non volatile solute having a molecular weight of 90 are to be dissolved in 97 5 g water in order to decrease the vapour pressure of water by 2 5 percent 1 25 3 12 5 2 18 4 9 61 Among the following that form a non ideal 67 68

Physical Chemistry
GeneralConsider the following statements regarding the solution of alkali metals and liquids ammonia 1 Rb dissolves in liquid ammonia giving deep blue colour II In concentrated solution the blue colour changes to bronze colour III Blue solutions are paramagnetic whereas bronze coloured solutions are diamagnetic The correct statement s is are II only I and II only II and III only I II and III

Physical Chemistry
Generalru to its mole fraction in sol Case I A solute is a volatile solid or liquid On the basis of his study of a number of binary solutions of In a solution the vapor pressure of a component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapor pressure of that component volatile components Raoult stated that in the pure state at the same temperature components A and B IX and XB are the mole fractions of the Let us consider a solution of two miscible volatile liquid two components A and B and p and pg are their vapor pressure in the pure state then according to Raoult s law PAA PA PB XB PB and