General Questions and Answers
Physical Chemistry
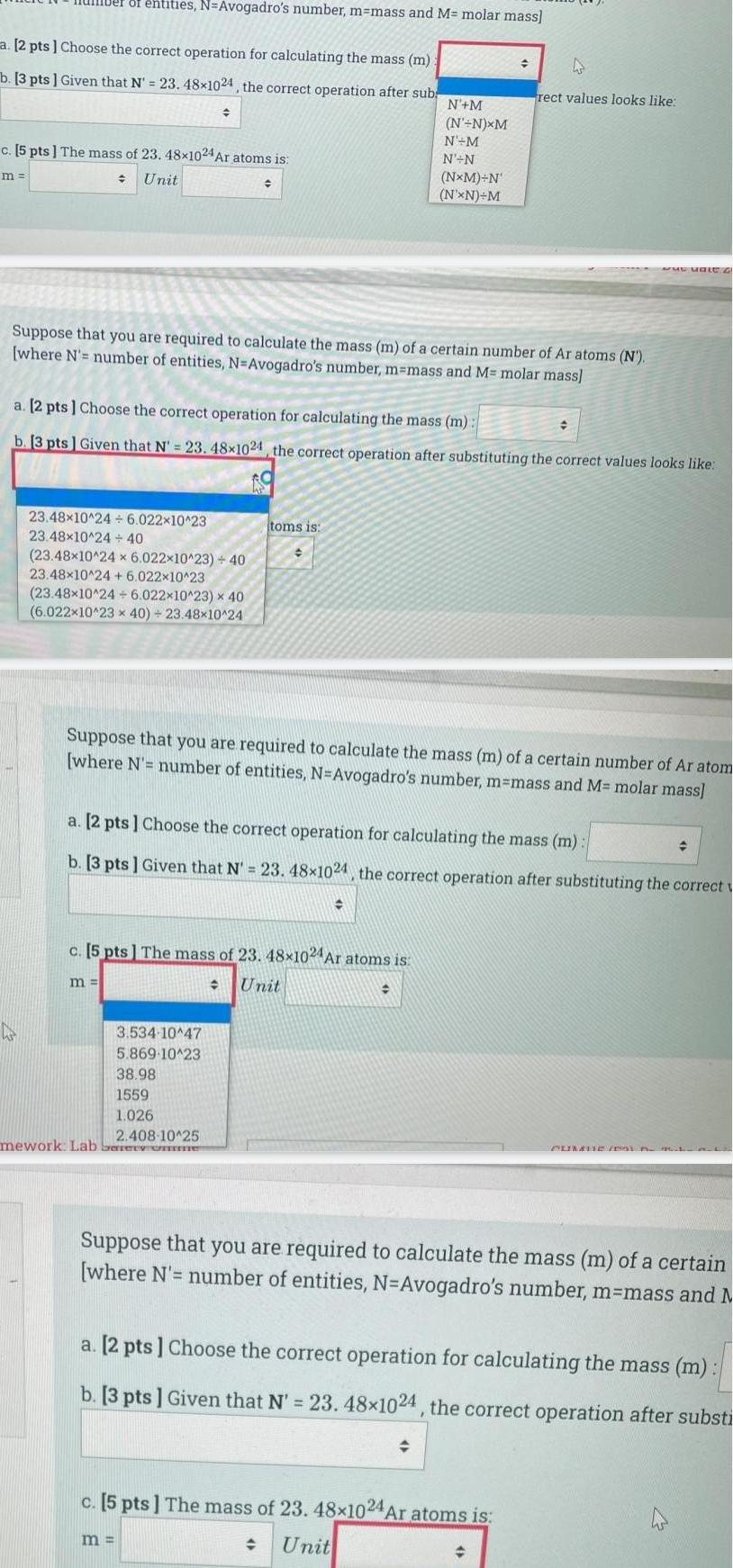
Generala 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48x1024 the correct operation after sub ber of entities N Avogadro s number m mass and M molar mass m c 5 pts The mass of 23 48x1024 Ar atoms is Unit 23 48x10 24 6 022 10 23 23 48x10 24 40 23 48x10 24 x 6 022x10 23 40 23 48x10 24 6 022x10 23 23 48x10 24 6 022x10 23 x 40 6 022x10 23 x 40 23 48x10 24 Suppose that you are required to calculate the mass m of a certain number of Ar atoms N where N number of entities N Avogadro s number m mass and M molar mass m a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substituting the correct values looks like 3 534 10 47 5 869 10 23 38 98 1559 1 026 2 408 10 25 mework Lab Sery one C h toms is m c 5 pts The mass of 23 48x1024 Ar atoms is Unit N M N N XM N M N N Suppose that you are required to calculate the mass m of a certain number of Ar atom where N number of entities N Avogadro s number m mass and M molar mass NxM N N XN M a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substituting the correct 4 rect values looks like c 5 pts The mass of 23 48x1024 Ar atoms is Unit www Male G Suppose that you are required to calculate the mass m of a certain where N number of entities N Avogadro s number m mass and M a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substi
Physical Chemistry
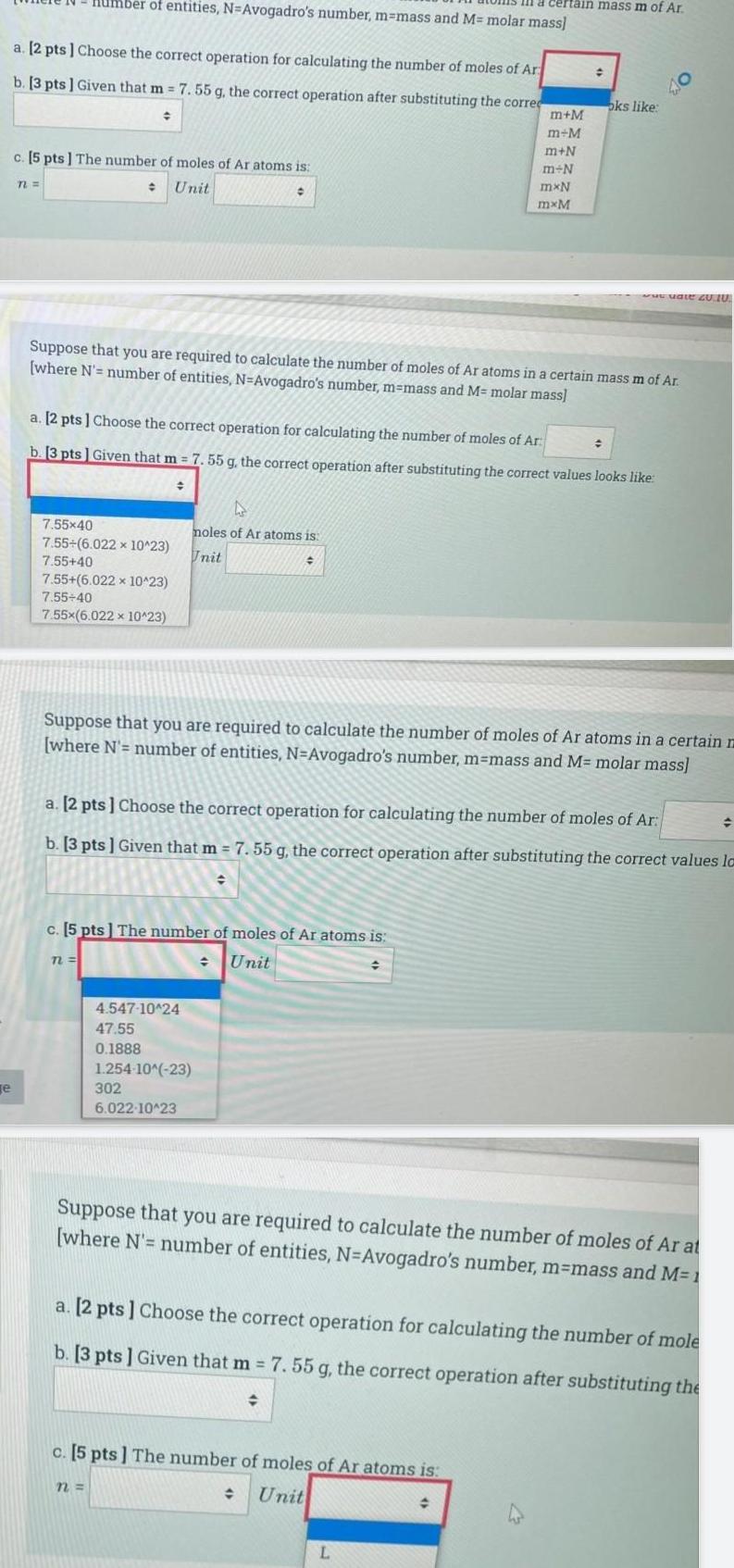
Generale a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correc c 5 pts The number of moles of Ar atoms is Unit n Imber of entities N Avogadro s number m mass and M molar mass 7 55x40 7 55 6 022 x 10 23 7 55 40 7 55 6 022 x 10 23 7 55 40 7 55x 6 022 x 10 23 72 Suppose that you are required to calculate the number of moles of Ar atoms in a certain mass m of Ar where N number of entities N Avogadro s number m mass and M molar mass a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correct values looks like c 5 pts The number of moles of Ar atoms is Unit D moles of Ar atoms is Unit 4 547 10 24 47 55 0 1888 n 1 254 10 23 302 6 022 10 23 Suppose that you are required to calculate the number of moles of Ar atoms in a certain m where N number of entities N Avogadro s number m mass and M molar mass certain mass m of Ar a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correct values la m M m M m N m N mxN mxM c 5 pts The number of moles of Ar atoms is Unit oks like L 40 www uate 20 10 Suppose that you are required to calculate the number of moles of Ar at where N number of entities N Avogadro s number m mass and M 1 a 2 pts Choose the correct operation for calculating the number of mole b 3 pts Given that m 7 55 g the correct operation after substituting the
Physical Chemistry
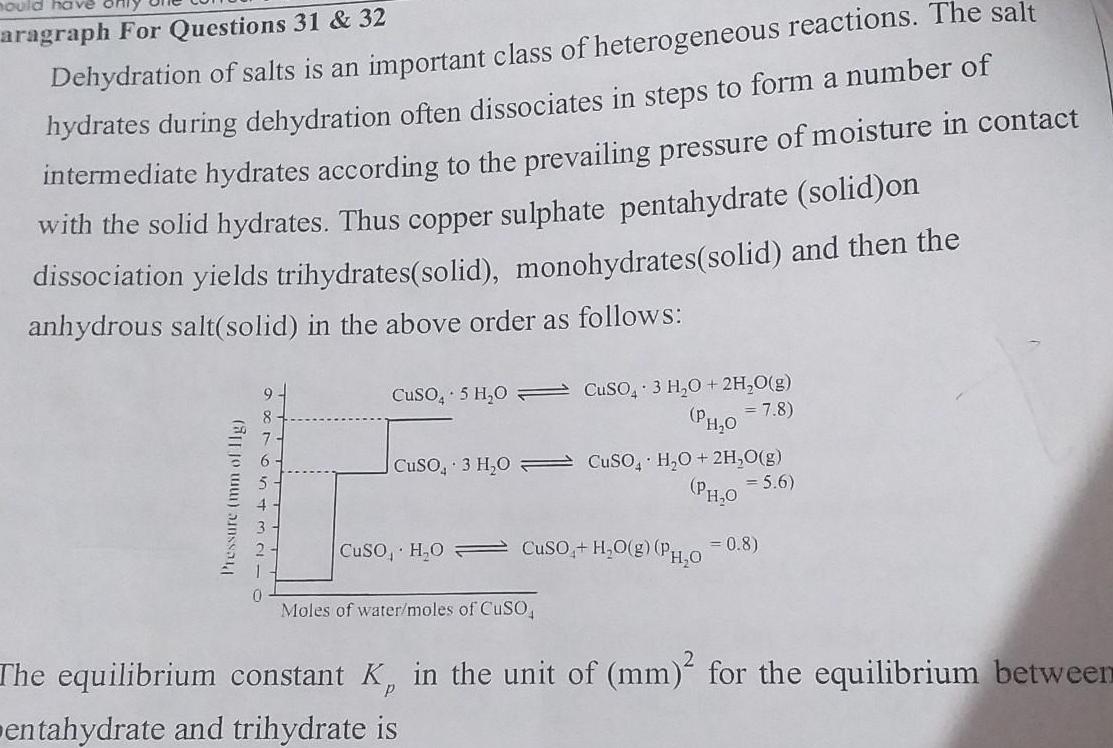
Generalhould have aragraph For Questions 31 32 Dehydration of salts is an important class of heterogeneous reactions The salt hydrates during dehydration often dissociates in steps to form a number of intermediate hydrates according to the prevailing pressure of moisture in contact with the solid hydrates Thus copper sulphate pentahydrate solid on dissociation yields trihydrates solid monohydrates solid and then the anhydrous salt solid in the above order as follows Pressure mm of lig CuSO4 5 H O CuSO3 H O SO4 H O 2H O g PH 0 5 6 CuSO H O CuSO4 H O g PH O 0 8 CuSO4 3 H O 2H O g PH 0 7 8 Moles of water moles of CuSO The equilibrium constant K in the unit of mm for the equilibrium between entahydrate and trihydrate is

Physical Chemistry
General3 pts each The atom with Z 16 a The atomic symbol is b The name of this atom is c This atom is in group d This atom is in period e This atom is classified as f The predicted ionic charge is 2 Ag sodium 6A 16 Na 2 iodine metalloid 2A 2 Ba nonmetal carbon barium C 5 S 1 silver 3 sulfur metal main gro 7A 17 22 27 37

Physical Chemistry
General3 04 A solution containing 4 M Cr O 2 M Cr shows a pH of 2 0 Calculate reduction potential 2 303 RT F of half cell reaction 0 06 E Cr 0 Cr 1 33 V A 0 5 V B 0 7 V C 1 05 V D 1 50 V

Physical Chemistry
GeneralHow to find atomic mass of an elemen t I know you will say Number of proton s Number of neutrons for example ox ygen atomic 16 8 8 Number of pro tons Number of neutrons but how the chlorine have mass of 35 5 amu I know also this very well Because of the average mass of their isotopes b ut I want to know that oxygen also have isotopes but its atomic mass Number of protons Number of neutro ns why in the case of oxygen we don t c alculate the average of the mass of thei risotopes

Physical Chemistry
Generaldata Based on the R2 factor and the percent relative uncertainty for the data what correlation can you make If a correlation is determined discuss possible R2 factor 0 98346808 P R U of the slope x 100 1 69

Physical Chemistry
GeneralThe temperature at which carbon reduced FeO into iron produces CO is 200 3 4 Correct Answer 4 Your Answer 3 4 Jour AG kJ 300 400 500 600 700 200 0 200 2Fe 0 2FeO Below temprature at point A Approx same temprature at point A Above than point A but below then point D temprature Above at point A temprature C 0 CO 20 0 200 400 800 1200 1600 2000 Temperature C POUR SELL UnationTZUEGUIUGIT rate 16 0

Physical Chemistry
Generalprovided as 37 Match the following Condition for the reaction to be favoured in forward direction COLUMN I A CO g H O 1 H CO aq AH 10kj mol B CO g 2H g CH OH g AH 91kj mol C N O g 2 NO g AH 57 2kJ mol D N g O g 2NO g AH 90 kJ mol A A p s B s C q r D q C A p B p s C q D q COLUMN II p Low temperature q High r s High B A p s B s C q r D q r D A p s B p s C q r D q temperature Low pressure pressure

Physical Chemistry
General16 2 mole of zinc is dissolved in HCl at 25 C The work done in open vessel is a 2 477 kJ c 0 0489 kJ b 4 955 kJ d None ogged by 2 C at constant TAL

Physical Chemistry
GeneralYour teacher has an addiction to Snickers bars food label below NUTRITION INFORMATION SERVINGS PER PACKAGE 1 SERVING SIZE 50g DI Per Average Quantities Per Serving Serve Per 100g ENERGY PROTEIN FAT Total Saturated CARBOHYDRATE Sugars SODIUM 1030kJ 12 4 4g 9 12 2g 17 4 5g 19 28 6g 9 25 6g 28 119mg 5 2020kJ 8 7g 24 0g 8 8g 56 5g 50 6g 236mg a Using the label and information provided how much energy in kJ is attributed to each of the major fo protein fats carbohydrates

Physical Chemistry
General9 The energy liberated when an excited electron returns to its ground state can have a any value from zero to infinity b only negative values c only specified positive values d none of the above ab 00

Physical Chemistry
General9 A solution contains Ag 0 14 Agtloin k ngta loilom KspAgI 8 5 10 17 M Ksp ng I 3 2 5 10 26 M Sri Chaitanya IIT Academy 35 27 12 15 Sr IPLCO JEE ADV 2014 P2 RPTA 16 Q Paper Calculate maximum concentration of iodide ion to be added from outsid which one of them gets precipitated almost completely A 8 5 10 MB 5 10 M C 1 7 x 10 M D 8 5 10 7 M What age of that metal ion is precipitated A 0 17 B 1 7 C 99 83 36 D 99 17

Physical Chemistry
General62 It s and S are solubility and solubility product of a sparingly soluble binary electrolyte respectively then 1 5 2 s 5 3 St JEO 20074 IN 200

Physical Chemistry
General50 Select the correct statement s 1 In Cr NH3 JCl3 the Werner primary valency is 3 ii Cr NH3 Cl3 is paramagnetic iii Co NH3 13 is a low spin complex 1 i ii and iii 3 ii and iii only 2 i and ii only 4 Only i

Physical Chemistry
General2 5 pts each Using the smallest possible whole numbers balance the following equation You need to drag and drop all correct coefficients including 1 As4S6 s O g As406 aq SO2 aq

Physical Chemistry
GeneralQ3 Metals have conductivity of the order of ohm cm 1 10 2 2 108 3 10 4 10 6 1 2 53 From NCERT 3 4

Physical Chemistry
GeneralCombustion of butane C4H10 the main component of lighter fluid is described by the chemical equation 2 CaHio 13 0 8 CO 10 H O In a particular case 84 5 grams of butane are formed by this reaction a How many moles of butane are formed b How many moles of O are consumed

Physical Chemistry
General13 You take 3mL of your unknown sample and 7mL water and mix them together The diluted sample gives an absorbance of 0 432 What is the concentration of the initial unknown In other words what was the concentration of your ORIGINAL solution

Physical Chemistry
General2Cu 41 Cu l2 12 12 2Na2 S O3 2Nal Na2 S406 If 100ml of CuSO4 Solution added to excess KI requires 50ml of 0 2MNa2 S2O3 The molarity of CuSO solution 0 05 M 0 1 M 0 2 M ion the following reactions took place 0 25 M A

Physical Chemistry
GeneralPick out the wrong statement 1 K Cr O7 reduces ferric sulphate to ferrous sulphate 2 Iron do not form amalgam 3 Permanent magnet is made by an alloy called Alnico 4 In the Lathanides ionic radius decreases from La 3 to Lu 3

Physical Chemistry
GeneralPre Medical Chemistry 185 131 500 mL of 0 2 M acetic acid are added to 500 mL of 0 30 M sodium acetate solution If the dissociation constant of acetic acid is 1 5 x 10 5 then pH of the resulting solution is 115 0 2 9 0 3 3 0 4 40

Physical Chemistry
Generaltry ALLEN and PZ are equal then 57 If the solubility of PbBr is S g molecules per litre will be considering 100 ionisation its solubility product 2 45 3 45 4 25 K PZ K PZ is 1 25

Physical Chemistry
GeneralSir please read the whole doubt I have tried to explain my doubt as much as I can so tha t I could get the correct explanation for it an d I hope that you will see my doubt and reso Ive it my doubt is that why tertiary alcohol i s more reactive than Primary and secondar y In the internet it was given that it is more reactive because of 3 alkyl halides and so th e carbocation formed will be stable and in p rimary and secondary alcohols carbocation won t be stable but sir this case will be valid if the mechanism is Sn1 but If the mechanis m is Sn2 then there will be no formation of c arbocation instead if the mechanism is Sn2 then Primary alcohol Will react faster due to less steric hindrance But what I think is that if there is tertiary alc ohol then it will follow Sn1 mechanism and i f there is Primary alcohol then it will follow Sn2 mechanism Then in that case we have to compare that whether sn1 will be faster o r sn2 will be faster in tertiary and Primary re spectively And then we can say whether ter tiary alcohol is more reactive or Primary alc ohol is more reactive But I don t know whet her sn1 is fast or sn2 Please tell whether I a m correct upto here or not and also that whi

Physical Chemistry
General10 5 M NaOH solution at 25 C is diluted 1000 times The pH of the resultant solution will 1 3 4 Correct Answor 2 Your Answer 1 Status incorrect be equal to 8 lie between 7 and 8 lie between 6 and 7 romain unchanged

Physical Chemistry
GeneralA certain vitamin extracted from plant sources has carbon and hydrogen in 8 1 mass ratio The percentage of oxygen is nearly 7 3 The compound gave no test for nitrogen or sulphur or any other element What should be the empirical formula of the compound A C30H4502 B C15H230 C C29H4503 D C 0H150

Physical Chemistry
Generalo 73 View in English When a quantity of electricity equal to that required to liberate 2 24 L of hydrogen at STP from 0 1 M aqueous H SO4 is passed then the mass of copper that will be deposited at cathode in electrolysis of 02 M solution of copper sulphate will bo At mass of Cu 63 5 1 4 orrect Answer 3 tatus unattempted Add to Bookmark List 159 g 3 18 g 6 35 g 12 70 g Full Paper 20 Q No Question Status Marks Time Solution

Physical Chemistry
Generalons continued 11 What is the oxygen binding capacity of hemoglobin in a blood sample that is 100 saturated with O and has a total hemoglobin value of 12 g dL a 17 mL O dL b 4 mL O dL c 8 mL O dL d 34 ml Old 513 pix Thr

Physical Chemistry
GeneralIn human body CO combines with H O to form carbonic acid which dissociates as H CO3 H HCO3 During fear and excitation the rate of respiration increases which results the decrease in the concentration CO in blood How pH of the human blood serum is affected during such a phenomena 1 decrease 2 remains constant 3 increases ENTRE 4 change in pH cannot be determined through given information

Physical Chemistry
GeneralA stone is thrown from the top of the cliff at a speed of 29 4 m s 1 in a direction 60 above the horizontal What is the time taken by the stone to return to the same level Consider g 9 8 m s A 2s B 3 s As

Physical Chemistry
GeneralConsider the reactions Baeyer s reagent X MCPBA Y H O Which of the following is correct X and Y are identical X and Y are enantiomers X and Y are diastereomers X and Y are positional isomers

Physical Chemistry
GeneralCorrect statements among the following are a Commercial bleaching solution contains 3 62 mass percentage of sodium hypochlorite in water b 35 v v solution of ethylene glycol an antifreeze is used in cars for cooling the engine c 1 5 parts per million of fluoride ions in water causes the tooth to become mottled d Mass and mole fraction are independent of temperature 1 a and c 3 a b and d 2 a and b 4 a b c and d

Physical Chemistry
GeneralTitration curves for 0 1 M solutions of three weak acids HA HA HA with ionization constants K K and K respectively with a strong base are plotted as shown in the figure Which of the following options is are true K K 2 A K C K K 12 10 PH of of 41 Volume of strong base added B pK pK D K K

Physical Chemistry
GeneralAir conditioners dry the air while they cool the air Suppose an air conditioner is used to cool a room that is 7 06 m x 11 66 m x 2 53 m when the outside temperature is 33 C At this temperature the vapor pressure of water is in grams is 37 7 torr The partial pressure of water in the air is 83 5 of the vapor pressure of water How much water removed from the air cach time the air from the room is cycled through the air conditioner mass of H O

Physical Chemistry
GeneralQ1 Manish got a mixture of camphor common salt and soil from his teacher The teacher asked him to separate camphor from the mixture Which technique can Manish use to separate camphor from the mixture easily filtration sublimation O evaporation O crystallization

Physical Chemistry
Generala For the reaction 3Fe A14Si5018 Fe cordierite 2Fe AlSi3012 4A12SiO3 5SiO Fe garnet sillimanite quartz AH 160 6 kJ mol AS 26 2 e u AV 164 8 cm Assuming V is not a function of T or P and that all minerals present are pure phases calculate the equilibrium for the above reaction using pure Fe end members of the cordierite and garnet solid solutions assumed to be Graph the phase diagram

Physical Chemistry
General23 Balanced chemical equation of combustion of ethane is represented as 2 C2H6 7 02 4 CO2 6 H2O Heat Energy Which of the following holds true out of the following 1 Point O2mole of ethane on combustion gives 3 mole of water 7 mole of oxygen is sufficient for combustion of 1 mole of ethane 88g of carbondioxide is produced when 2 mole of ethane is burnt in the presence of oxygen 18 g of water is produced when 30g of ethane is burnt

Physical Chemistry
General41 lodine molecule dissociates into atoms after absorbing light of 4500 If one quantum of radiation is absorbed by each molecule the kinetic energy per iodine atom is given by x x 10 J in scientific notation What is the value of x yl Use h 6 6 x 10 34 Js c 3 108 ms 1 and X N 6 x 1023 bond energy of l 240 kJ mol 1 A

Physical Chemistry
GeneralPart B Calculate the following 1 How many atoms are in a 3 5 g sample of sodium Na 2 How many grams are in 6 53 x 1022 molecules of Alcohol C H6O 3 In number two question how many atoms of Hydrogen present in Alcohol 4 In a chemical reaction of Iron and Chlorine produce a product of Iron III Chloride Fe Cl FeCl3 Calculate the limiting reactant 5 Calculate the Molecular Weight of C8H10N 402

Physical Chemistry
GeneralTi is brown while Ti4 is colourless because A Ti4 is unaffected by the crystal field Ti2 has a 3d configuration Ti4 has a 3d configuration Ti4 cannot absorb the visible light since it is smaller than Ti

Physical Chemistry
General40ml of minture of G H1 2 and co is mined with 100 ml of 0 gas and the minture is emploded The residual gas occupied woy ml and are passed through KOH Solution the volume becomes 48 ml Find composition of original minture when these

Physical Chemistry
GeneralIn a Hard Water Sample MgSO4 1 2 ppm Then the permanent h water is Option 1 2 3 ppm Option 2 2 ppm Option 3 4 ppm Option 4

Physical Chemistry
GeneralI saw an method in byjus video about how t o balance chemical equations through alge braic method but when I tried to solve a part icular equation i wasnt able to balance it th ose equations are H2 Cl2 HCI H2 N2 NH3 PLEASE PROVIDE A WRITTEN SOLUTION O NA PAPER WITH CALCULATION ONLY THR OUGH ALGEBRAIC METHOD

Physical Chemistry
GeneralWhich of the following statements are incorrect Options 1 Hydrogen like alkali metals is electropositive 2 Like alkali metals hydrogen can show an oxidation state of 1 in its compo 3 Like alkali metals hydrogen can act as a strong reducing agent 4 Hydrogen shows an oxidation state of 1 in covalent hydrides

Physical Chemistry
GeneralHypochlorous acid HOCI a weak acid dissociates partially to form a hydrogen ion H and its conjugate base hypochlorite ion OCI in water Find the fraction of non dissociated HOCI with respect to the total concentration of HOCI and OCI at different pH values Plot the fraction of HOCI and OCI with respect to the total concentration HOCI OCI vs the pH of the solution Use pH values 3 6 9 and 12 for the plot Hint Define a HOCI HOCI OCI and a OCI HOCI OCI Plot a and a vs pH

Physical Chemistry
GeneralResistance of 0 2 M solution of an electrolyte is 50 2 The specific conductance of the solution is 1 4 S m The resistance of 0 5 M solution of the same electrolyte is 280 2 The molar conductivity of 0 5 M solution of the electrolyte in S m mol is 2 A 5 x 104 C 5 10 B D JEE Main 2014 5 x 10 3 5 x 10

Physical Chemistry
GeneralAverage Rate A R At A P At Then how can we include that 1 2 here when average rate is asked Please explain Sir Mam In a reaction 2HI g H g 1 g the concentration of HI decreases 0 6 M to 0 5 M in 10 minutes The average rate reaction during this interval is 0 05 M min 1 0 005 M min 1 0 01 M min 1 0 001 M min 1 Solution Answer 2 2HIH g 1 g 1 A HI A H A 12 2 At At At 1 0 6 0 5 0 005 M min 1 Average rate reaction

Physical Chemistry
General7 in which pair most efficient packing 1 point is present Ohcp and ccp Ohcp and bcc bcc and ccp Occp and simple unit cell 8 What is effect of frenkel defect on density of ionic solid increase O decrease remain unchanged not relationship between density and defect 9 In Schottky defect 1 point electron are trapped in some lattice site 1 point ion moves to interstitial position between the lattice point

Physical Chemistry
General0 6 g of a solute is dissolved in 0 1 L of a solvel develops an osmotic pressure of 1 23 atm at 27 C The molecular weight of the solute is 2 120 0 g mol 1 4 None of these 1 149 5 g mol 1 3 430 0 g mol

Physical Chemistry
General3 50 ml of 0 2 M NH 50 ml of 0 2M HCI 4 50 ml of 0 2 M NH 50 ml of 0 IM HCI 3 011 x 1022 atoms of an element weight 1 15 g 73 The atomic mass of the element is 1 2 3 3 4 6 For the reaction A 2B C 8 mole of A and 74 2 23 4 46 3 50 ml of 0 2 M NH 50 ml of 0 2M HCI 4 50 ml of 0 2 M NH 50 ml of 0 IM HCI 3 011 1022 1 15 a m 1 2 3 3 4 6 2 23 4 46 3A 2BC fer R A