General Questions and Answers

Physical Chemistry
General2 Absolute temperature is the tempera which a all molecular motion ceases b bolume becomes zero c mass becomes zero d none of the above

Physical Chemistry
General3 1 2 If H SO ionises as H SO4 2H O 2H O SO then total number of ions produced by Molar and 1L aqueous H SO4 will be 2 3 01x10 2 4 1 8x10 4 1 1 9 03 10 3 6 02 10 A micelle formed during the cleansing action by soap is

Physical Chemistry
GeneralDuring the experiment a student determined that 19 50 ml NaOH solution from problem 2 was used to titrate a 6 00 mL of a vinegar sample Determine the concentration as mass of acetic acid HC H30 in the vinegar density if vinegar 1 02 g mL MM acetic acid 60 05 g mol

Physical Chemistry
General1 Ba 2 Ba 3 Ba The pH value of decinormal solution of NH OH which is 20 ionized is 1 13 30 2 14 70 3 12 30 4 12 95 Among the following the compound that is both paramagnetic and coloured is 1 K Cr O 2 KMnO 3 COSO 4 K Cu CN 141 4 B

Physical Chemistry
Generalplatinum wire loop of the salt with concentrated HCI When he takes a small amount of this J KA TOPPER paste and keeps it in a non luminous Bunsen flame the colour of the flame changes to grassy green He should therefore conclude that the metal is 1 Barium 2 Calcium 3 Potassium 4 Strontium

Physical Chemistry
Generalgas absorbs photon of wavelength 345 nm and emits two wavelengths If one of the emission is at 680nm the other at A 1035nm B 325nm C700nm D 518nm A

Physical Chemistry
Generald obeyance of Raoult s law Among the following mixtures dipole dipole as the major interaction is present in a benzene and ethanol b KCl and water acetonitrile and acetone d benzene and CC14

Physical Chemistry
GeneralThe alkali metals form salt like hydrides by the direct synthesis at elevated temperature The thermal stability of these hydrides decreases in which of the following orders LiH NaH KH RbH CsH CsH RbH KH NaH LiH KH NaH LiH CsH RbH NaH LiH KH RbH CsH

Physical Chemistry
Generala 4 b 3 c 2 139 6x10 mole K Cr O reacts completely with 9x 10 3 mole X to give XO3 and value of n is Doubt a 1 b 2 c 3 d None of these 140 What weight of H C 04 2H 0 mol wt 126 should be dissolved in water to prepare 250 ngent

Physical Chemistry
General2 5 g acetic acid CH3COOH is dissolved in 75 g of benzene Calculate the molality of this solution A 0 555 m B 0 666 m 0 444 m D 0 777 m of

Physical Chemistry
General9 How many litre of oxygen at STP is required to bum 60 g C H 1 22 4 L 2 11 2 L 3 22 4x7 L 4 8 5 L D For the formation of 3 65 g of HCI gas what volume

Physical Chemistry
General1 6 1 6 147 What is the molar solubility of Mn OH 2 Ksp 4 5 x 10 14 in a buffer solution containing and NH3 K 1 8 x 10 5 b 1 38 x 10 4 equal amounts of NH a 3 0 10 4 c 1 38 10 148 Find moles of NH4Cl required to prevent Mg OH from precipitating in d 7 3 10 4

Physical Chemistry
Generalor which reaction s given fact s is are correct H S H O H O HS Kc is ionization constant of acid H O H O H O OH Kc is ionic product of water 1 CH NH H O CH NH3 OH Kc is ionization constant of base 2 O Cu 4NH Cu NH3 2 Kc is stability constant of complex 2 4

Physical Chemistry
GeneralThe concentration of an organic compound in chloroform is 6 15 6 15 g per 100mL of solution A portion of this solution in a 5cm polarimeter tube causes an observed rotation of 1 2 What is the specific rotation of the compound EAMCET 2009 a 12 b 3 9 c 39 d 61 5

Physical Chemistry
General3 21 molecules 4 101 molecules 4 If the weight of metal oxide is x g containing yg of oxygen the equivalent weight of metal will be 2 E 1 E 8x y E 4 E 8 y x X 8 x y y

Physical Chemistry
GeneralNumber of Fe atoms in 100 g Haemoglob contains 0 33 Fe Atomic mass of Fe 5 1 0 035 1023 3 3 5 1023 An organic compound 2 35 4 7 x 10 containing C and H ga

Physical Chemistry
General3 6 025 x 100 molecules of acetic acid are present in 500 ml of its solution The concentration of solution is 1 0 002 M 2 10 2 M 3 0 012 M 4 0 001 M How many litre of oxygen at STP is required to burn

Physical Chemistry
Generald 5 0 x 10 6 38 On adding AlCl3 to water a the ionisation of water increases b the ionisation of water decreases c the ionisation of water remains constant d the ionic product of water increases What it

Physical Chemistry
GeneralSelect the incorrect statement Potassium carbonate on reaction with hot steam gives KOH and CO2 Potassium serperoxide reacts with sulphur t give potassium sulphide Black ash obtained by the conversion of salt cake Na2SO4 a solid residue with 45 of Na CO3 Sodium chloride is slightly hygroscopic

Physical Chemistry
GeneralSodium chloride is soluble in water but not in benzene because in water and AHAH in benzene in benzene in benzene in benzene 1 AH AH 2 AH 3 AH 4 AH AH AH AH Schon Schon in water and AH AH Las in water and AH Schon AH Laming in water and AH AH Lattice sergy

Physical Chemistry
General5 1 5 gm of an unknown gas at 127 C occupies the same volume as 9 6 gm ozone gas at 27 C at the same pressure The molar mass of unknown gas is A 10 gm mol C 15 gm mol B 5 gm mol D 20 gm mol

Physical Chemistry
GeneralGiven the numbers 161 cm 0 161 cm 0 016 The number of significant figures for the numbers is 1 3 3 and 4 respectively 2 3 4 and 4 respectively 3 3 4 and 5 respectively 4 3 3 and 3 respectively

Physical Chemistry
GeneralMagnetic moments of V Z 23 Cr Z 24 Mn Z 25 are x y z Hence A x y z B C x z y skipped x y z D z y x

Physical Chemistry
General1 m n 2 n m 3 2 4 2 How many carbon atoms are present in 0 35 mole of C H O AAJ KA TOPPER Given N 6 023x10 1 1 26x10 carbon atoms 3 1 26x10 carbon atoms Which of the followfou conficiratione 2 1 26 10 carbon atoms 4 1 26 10 carbon atoms forms on e netobortial no play only

Physical Chemistry
General6 gram atom contains Natoms A One mole of sucrose reacts completely with oxygen produces 268 8 litre of carbon dioxide at STP R Amount of oxygen required for reaction is 268 8 litre 7 A In the reaction

Physical Chemistry
GeneralWhich of the following statements about chemisorption is not applicable O O O It involves chemical forces between adsorbent and absorbate OIt is irreversible in nature OIt involves high heat of adsorption OIt involves low activation energy

Physical Chemistry
GeneralThe amount of zinc required to produce 1 12 ml of H at STP on treatment with dilute HCI will be 1 65 g 2 0 065 g 3 32 5 10 g 4 6 5 g

Physical Chemistry
General38 An element X has the following isotopic composition sex 90 57Xx 8 x 2 0 The weighted average atomic mass of the naturally occurring element X is closest to 1 56 14 amu 3 60 amu 100 of 39 2 56 8 amu 4 55 amu and 64 of rynen

Physical Chemistry
GeneralThe number of mole of nitrogen in one litre of air containing 10 nitrogen by volume under standard conditions is 1 0 03 mole 3 0 186 mole 2 2 10 moles 4 4 46 10 3 mole

Physical Chemistry
GeneralThe graph of compressibility factor Z vs P for one mode of a real gas is plotted at constant temperature 273 K If the slope of graph at very dZ 1 IS atm 1 the volume of one molecule of real gas in cm is x x 10 22 Find x Take N 6 1023 dp 10 high pressure

Physical Chemistry
GeneralA cylinder contains either ethylene or propylene 12 ml of gas required 54 ml of oxygen for complete combustion The gas is 1 Ethylene 2 Propylene 3 1 1 mixture of two gases 4 1 2 mixture nific found

Physical Chemistry
General3 40 kcal 4 76 kcal 18 The difference between AH and AE for the reaction 12CO g 6H O at 25 C 2 3 72 kJ 2CH 150 0 in kJ is 1 7 43 kJ 31 372k1
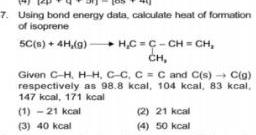
Physical Chemistry
General7 Using bond energy data calculate heat of formation of isoprene 5C s 4H g H C C CH CH CH Given C H H H C C C C and C s C g respectively as 98 8 kcal 104 kcal 83 kcal 147 kcal 171 kcal 1 21 kcal 3 40 kcal 2 21 kcal 4 50 kcal

Physical Chemistry
GeneralHydroxyl amine reduces iron III according to following equation NH OH Fe SO4 3 N g H O FeSO4 H SO4 Which statement is correct A n factor for Hydroxyl amine is 1 B equivalent weight of Fe SO is M 2 C 6 meq of Fe SO is contained in 3 millimo les of ferric sulphate

Physical Chemistry
GeneralBoard Competitive Exams 18 If the weight of metal chloride is x gram containing y gram of metal the equivalent weight of metal will be 1 E x35 5 3 E y x y x35 5 2 E 4 E 8 y x 8 x y y

Physical Chemistry
General80 kJ of heat is given to 36 g of water Then the a b 80 number of H and OH ions are 1 2044 10 4 each 80 number of water molecules that remain in solution is 36 18x6 02 x 1023x80 H 80 36 6 02 x 10 OH numbers of H and OH ions are 1 2044 x 10 24 80 c the ratio of H and OH d each ions

Physical Chemistry
GeneralA bowman is shooting arrows at a target Which of the following demonstrates high accuracy but low precision The bowman consistently hits to the left of the target The bowman consistently hits to the right of the bullseye 1 poin The bowman consistently hits around the target but never hits the bullseye The bowman consistently hits the bullseye The bowman consistently misses the target and hits a tree in the same spot
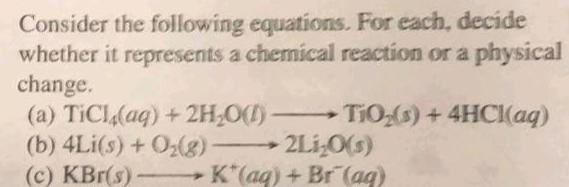
Physical Chemistry
GeneralConsider the following equations For each decide whether it represents a chemical reaction or a physical change TiO s 4HCl aq a TiCl aq 2H O b 4Li s O g c KBr s 2Li O s K aq Br aq

Physical Chemistry
General20 The values of four weak acids W X Y and Z are listed below Which acid is weakest Explain W X Y Z 21 What is the Br nsted Lowry conjugate base of Write an equation to illustrate your answer 22 Three aqueous solutions of nitric acid are listed below W X Y What is the order of increasing pH of these solutions 23 Four aqueous solutions are listed below W X

Physical Chemistry
GeneralWhich of the solvent given in figure could be used to discriminate the acidities of PH3 pka 27 and GeH4 pKa 25 Fluorosulfuric acid H SO F Hydrofluoric acid HF 20 Sulfuric acid H SO Methanoic acid HCOOH 10 Ethanoic acid CH COOH Ethanol CH CH OH Water H O Dimethylsulfoxide DMSO 2 points Ammonia NH 20 30 0 10 Effective pH in water 40

Physical Chemistry
GeneralFour elements arbitrarily labelled A 1 point B C and D have electronegativities 3 8 3 3 2 8 and 1 3 respectively Place the compounds AB AD BD and AC in order of increasing covalent character

Physical Chemistry
General4 All triangular units 3 All tetrahedral units A 0 01 M ammonia solution is 5 ionized its pH will be 1 11 80 2 10 69 3 7 22 4 12 24 Which one of the following compounds is different from the rest in terms of undergoin hydrolysis to form simpler compounds 1 Sucrose 2 Maltose 3 Lactose 4 Glucose

Physical Chemistry
GeneralCHEMISTRY Match the compound with the metal for which it is used for the process of extraction i NaCN ii lodine iii Cryolite a Titanium b Aluminium c Silver ore 1 i c ii a iii b 3 1 a ii c iii b Salicylic acid is produc 2 i c ii b iii a 4 1 b ii a iii c when phonol in alcoholic KOH is treated with

Physical Chemistry
General14 If 50 ml of M 50 NaOH 20ml of M 40 KOH and 30 ml of M 60 CSOH are mixed then OH concentration in resultant solution will be 1 2 10 2 M 3 2 0 M 2 1 x 10 M 4 5 10 2 M

Physical Chemistry
GeneralA AgNO3 solution containing 0 00739 g of AgNO3 per gram of water is electrolyzed between Ag electrodes During the experiment 0 078 g silver was deposited on the cathode At the end of the experiment anode solution contains 23 14 g of silver nitrate What is the transport number of silver ion Atomic weight of silver and molecular weight silver nitrate are 108 and 170 respectively 3 3

Physical Chemistry
General11 Phosphoric acid H3PO4 is a weak electrolyte because it A forms a number of different ions B dissociates incompletely in solution C consists of more than two atoms rotons to solution

Physical Chemistry
GeneralTwo solutions of H SO4 are prepared with different composition Solution A 500 mL of 1 M aq H SO4 having density 1 2 g mL Solution B 100 mL of 2 M aq H SO4 having density 1 4 g mL Select the correct statement If both solutions A and B are mixed then molarity of final solution is 2 33 M If both solutions A and B are mixed then w w of HOSO is 10 72 in final solution

Physical Chemistry
General8 Which property of water makes it well suited for the transport of gases and electrolytes through the human body F Its low molecular weight makes it an easy substance to pump G Its high polarity makes it an excellent solvent of many substances H Its high specific heat makes it resistant to temperature change 3 Its relative chemical stability makes it an excellent energy source

Physical Chemistry
General26 An athlete takes 100 g of glucose of energy equivalent to 1560 kJ How much amount of energy is uptaken by 1 g molecule of glucose 2 2808 kJ 4 28 08 kJ 1 15 6 kJ 3 1560 kJ

Physical Chemistry
General42 At 27 C latent heat of fusion of a compound is 2930 J mol Entropy change is 1 9 77 J mol K 3 9 07 J mol K 2 10 77 J mol K 4 0 977 J mol K