In the world of organic compounds, one substance that stands out is benzoic acid. This versatile compound has a wide range of applications, from food preservation to the synthesis of other organic substances. In this article, we will explore the structure, properties, uses, and production of benzoic acid, as well as its chemical reactions and frequently asked questions.
What is Benzoic Acid?
Benzoic acid, with the chemical formula C6H5COOH, is a white or colorless solid organic compound. It is the simplest aromatic carboxylic acid, consisting of a carboxyl group (-COOH) attached to a benzene ring. The name “benzoic acid” is derived from “gum benzoin,” which was once its only source. Today, benzoic acid is found naturally in many plants and serves as an intermediate in the biosynthesis of various secondary metabolites.
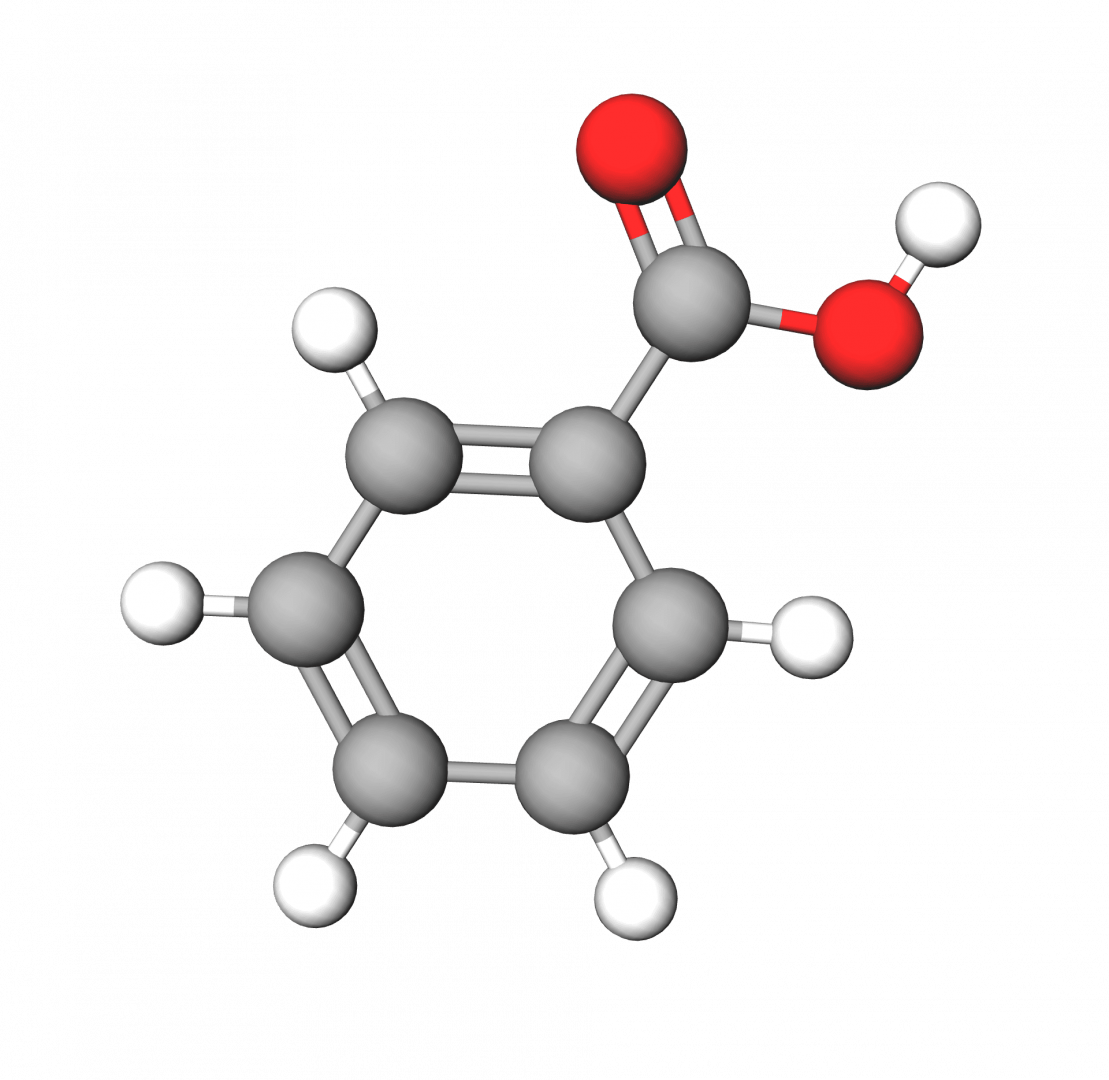
Benzoic Acid Structure
The structure of benzoic acid consists of a benzene ring (C6H6) with a carboxyl group (-COOH) attached to it. The benzene ring is a hexagonal ring of carbon atoms, with alternating single and double bonds. The carboxyl group consists of a carbon atom double-bonded to an oxygen atom, and single-bonded to an oxygen atom that is also bonded to a hydrogen atom.

Benzoic Acid Formula
The chemical formula of benzoic acid is C6H5COOH. This formula represents the composition of benzoic acid, indicating that it is composed of six carbon atoms (C6), five hydrogen atoms (H5), and two oxygen atoms (O). The COOH portion represents the carboxyl group.
Properties of Benzoic Acid
Chemical Data
Here are some key chemical data for benzoic acid:
- Chemical formula: C6H5COOH
- Molar mass: 122.12 g/mol
- Appearance: White or colorless crystalline solid
- Odor: Faint, pleasant odor
- Density: 1.2659 g/cm3 (15 °C)
- Melting point: 122 °C (252 °F; 395 K)
- Boiling point: 250 °C (482 °F; 523 K)
- Solubility in water: 1.7 g/L (0 °C), 56.31 g/L (100 °C)
Chemical Data Table
| Property | Value |
|---|---|
| Molar mass | 122.123 g/mol |
| Density | 1.2659 g/cm³ (15 °C) |
| Melting point | 395 K |
| Boiling point | 523 K |
| pKa | 4.202 |
Physical Properties
Benzoic acid is a colorless crystalline solid with a faint, pleasant odor. It has a density of 1.2659 g/cm3 at 15 °C. The melting point of benzoic acid is 122 °C, and its boiling point is 250 °C. Benzoic acid is sparingly soluble in cold water, with a solubility of 1.7 g/L at 0 °C. However, its solubility increases significantly with temperature, reaching 56.31 g/L at 100 °C. Benzoic acid is more soluble in organic solvents such as acetone, benzene, and alcohol.

Chemical Properties
Benzoic acid exhibits various chemical properties. It is a weak acid with a pKa value of 4.2. This means that in an aqueous solution, benzoic acid partially dissociates, releasing hydrogen ions (H+) and forming benzoate ions (C6H5COO-). Benzoic acid can react with bases to form salts, such as sodium benzoate (C6H5COONa).
Uses of Benzoic Acid
Benzoic acid has a wide range of uses in various industries. Here are some of its primary applications:
- Food preservative: Benzoic acid and its salts, such as sodium benzoate, are widely used as food preservatives. They inhibit the growth of mold, yeast, and bacteria in acidic foods and beverages.
- Cosmetics: Benzoic acid is used as an ingredient in cosmetic products like lipsticks and face wash creams. It acts as a preservative and helps extend the shelf life of these products.
- Industrial synthesis: Benzoic acid serves as an important precursor for the industrial synthesis of various organic substances. It is used in the production of phenol, which is further used in the synthesis of plastics, nylon, and other materials.
- Pharmaceutical applications: Benzoic acid has antimicrobial properties and is used in ointments to prevent or treat fungal skin diseases.
- Chemical synthesis: Benzoic acid is used as a starting material in the synthesis of various organic compounds. It can undergo reactions like esterification, halogenation, and sulfonation to form different derivatives.
Benzoic Acid Melting Point
The melting point of benzoic acid is 122 °C (252 °F; 395 K). This is the temperature at which benzoic acid changes from a solid to a liquid state. The melting point can vary slightly depending on the purity of the sample. It is important to note that the melting point is a characteristic property of a substance and can be used to identify and differentiate it from other compounds.
The Molecular Mass of Benzoic Acid
The molar mass of benzoic acid is 122.12 g/mol. This value represents the mass of one mole of benzoic acid molecules. It is calculated by adding up the atomic masses of all the atoms in the chemical formula (C6H5COOH). The molar mass is an essential parameter used in various calculations, such as determining the amount of benzoic acid needed for a reaction or analyzing its concentration in a solution.

Production of Benzoic Acid
Industrial Preparation
In an industrial setting, benzoic acid is produced by the partial oxidation of toluene with oxygen. This process utilizes cobalt or manganese naphthenates as catalysts. The reaction proceeds in high yield and uses readily available materials.
Laboratory Synthesis
In a laboratory, benzoic acid is synthesized mainly for its educational value. The compound is purified by recrystallization from water due to its high solubility in hot water and poor solubility in cold water, yielding around 65% of the product.
By Hydrolysis
Benzonitrile and benzamide, like other nitriles and amides, can be hydrolyzed to benzoic acid under acidic or basic conditions.
From Grignard Reagent
The Grignard reagent, bromobenzene, can be converted to benzoic acid via carboxylation of the intermediate phenylmagnesium bromide.
Oxidation of Benzyl Compounds
Benzyl alcohol, benzyl chloride, and all benzyl derivatives can be readily oxidized to benzoic acid.
Chemical Reactions Related to Benzoic Acid
Benzoic acid can undergo several chemical reactions, leading to the formation of different compounds. Here are some notable reactions:
- Formation of Salt: Benzoic acid reacts with bases, such as sodium hydroxide, to form salts like sodium benzoate.
- Formation of Esters: Benzoic acid can react with alcohols in the presence of a dehydrating agent, such as sulfuric acid, to form esters. For example, the reaction between benzoic acid and ethanol produces ethyl benzoate.
- Formation of Acid Halides: Benzoic acid reacts with compounds like thionyl chloride or phosphorus pentachloride to form acid halides, such as benzoyl chloride.
- Sulfonation: Benzoic acid can be sulfonated by reacting with fuming sulfuric acid, leading to the introduction of a sulfonic acid group onto the benzene ring.
- Nitration: Benzoic acid can undergo nitration reactions, where a nitro group (-NO2) is introduced onto the benzene ring.
- Halogenation: Benzoic acid can be halogenated by reacting with halogens, such as chlorine, in the presence of a catalyst. This leads to the substitution of hydrogen atoms on the benzene ring with halogen atoms.
- Decarboxylation of Benzoic Acid: Heating benzoic acid with soda lime (a mixture of sodium hydroxide and calcium oxide) leads to the removal of the carboxyl group, resulting in the formation of benzene.
- Dehydration Reaction: Benzoic acid can undergo a dehydration reaction in the presence of dehydrating agents, such as sulfuric acid, resulting in the removal of water and the formation of aromatic compounds.
- Reduction of Benzoic Anhydride: Benzoic anhydride can be reduced to benzoic acid by reacting it with reducing agents like sodium borohydride.
- Controlled Oxidation of Benzyl Alcohol: Benzyl alcohol can be oxidized to benzoic acid by treating it with oxidizing agents like potassium permanganate or chromic acid.
Frequently Asked Questions About Benzoic Acid
Why is benzoic acid soluble in hot water and not in cold water?
The solubility of benzoic acid increases with temperature. At 0 degrees Celsius, the solubility of benzoic acid is 1.7 grams per liter. However, when heated to 100 degrees Celsius, the solubility increases to 56.31 grams per liter.
Is benzoic acid harmful to use?
While benzoic acid is generally regarded as safe for use in foods and cosmetics, excessive consumption or exposure can lead to skin irritation and digestive issues. It’s always advisable to use products containing benzoic acid as directed and avoid excessive consumption.
What are the medicinal uses of benzoic acid?
Benzoic acid is often used in ointments and creams for the treatment of fungal skin diseases. It’s also a common ingredient in some over-the-counter topical antiseptics and decongestants.
How can benzoic acid be prepared?
Benzoic acid can be prepared through several methods, including the oxidation of toluene, the hydrolysis of benzamide and benzonitrile, and the oxidation of benzyl compounds.
Which acid is stronger benzoic acid or acrylic acid?
Acrylic acid is a stronger acid than benzoic acid. This is because the carboxyl group in acrylic acid is attached to a carbon-carbon double bond, which is more electron-withdrawing than the benzene ring in benzoic acid.
Are all amino acids weaker acids than benzoic acid?
The acidity of an amino acid depends on its structure, particularly the nature and location of its side chains. While most amino acids are weaker acids than benzoic acid, certain amino acids with acidic side chains, such as glutamic acid and aspartic acid, can exhibit stronger acidity.
Is benzoate a polar or nonpolar molecule?
Benzoate, the anion derived from benzoic acid, is a polar molecule. This is because the distribution of electrons in the benzoate ion is not symmetric, leading to areas of partial positive and negative charge.
Is benzoic acid ionic or covalent?
Benzoic acid is a covalent compound. This is because it is composed of nonmetals, and the bonds between nonmetals are typically covalent, where electrons are shared rather than transferred.
Solved Examples on Benzoic Acid
Example 1: Calculate the molar mass of benzoic acid.
Solution: The molar mass of benzoic acid is calculated by adding up the atomic masses of all the atoms in the chemical formula. The atomic masses are: C = 12.01 g/mol, H = 1.01 g/mol, and O = 16.00 g/mol.
Molar mass of benzoic acid = (6 * 12.01) + (5 * 1.01) + 16.00 + 16.00 + 1.01 = 122.12 g/mol
Example 2: What is the melting point of benzoic acid?
Solution: The melting point of benzoic acid is 122 °C (252 °F; 395 K). This is the temperature at which benzoic acid changes from a solid to a liquid state.
Example 3: How is benzoic acid used in the food industry?
Solution: Benzoic acid and its salts are widely used as food preservatives in the food industry. They inhibit the growth of mold, yeast, and bacteria in acidic foods and beverages. Some examples of foods that may contain benzoic acid as a preservative include pickles, jams, carbonated drinks, and salad dressings.
How Kunduz Can Help You Learn Benzoic Acid?
At Kunduz, we are committed to providing you with comprehensive and accessible learning materials. Our goal is to empower you with the knowledge and skills you need to succeed in your academic journey. Whether you are studying chemistry, biology, or any other subject, we are here to support you every step of the way. Explore our resources and let us be your trusted companion in learning.
Remember, understanding the properties, uses, and production of benzoic acid is just the beginning. There is so much more to discover in the fascinating world of organic compounds. So, keep exploring, keep learning, and let Kunduz be your guide!
