Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.
Physical Chemistry
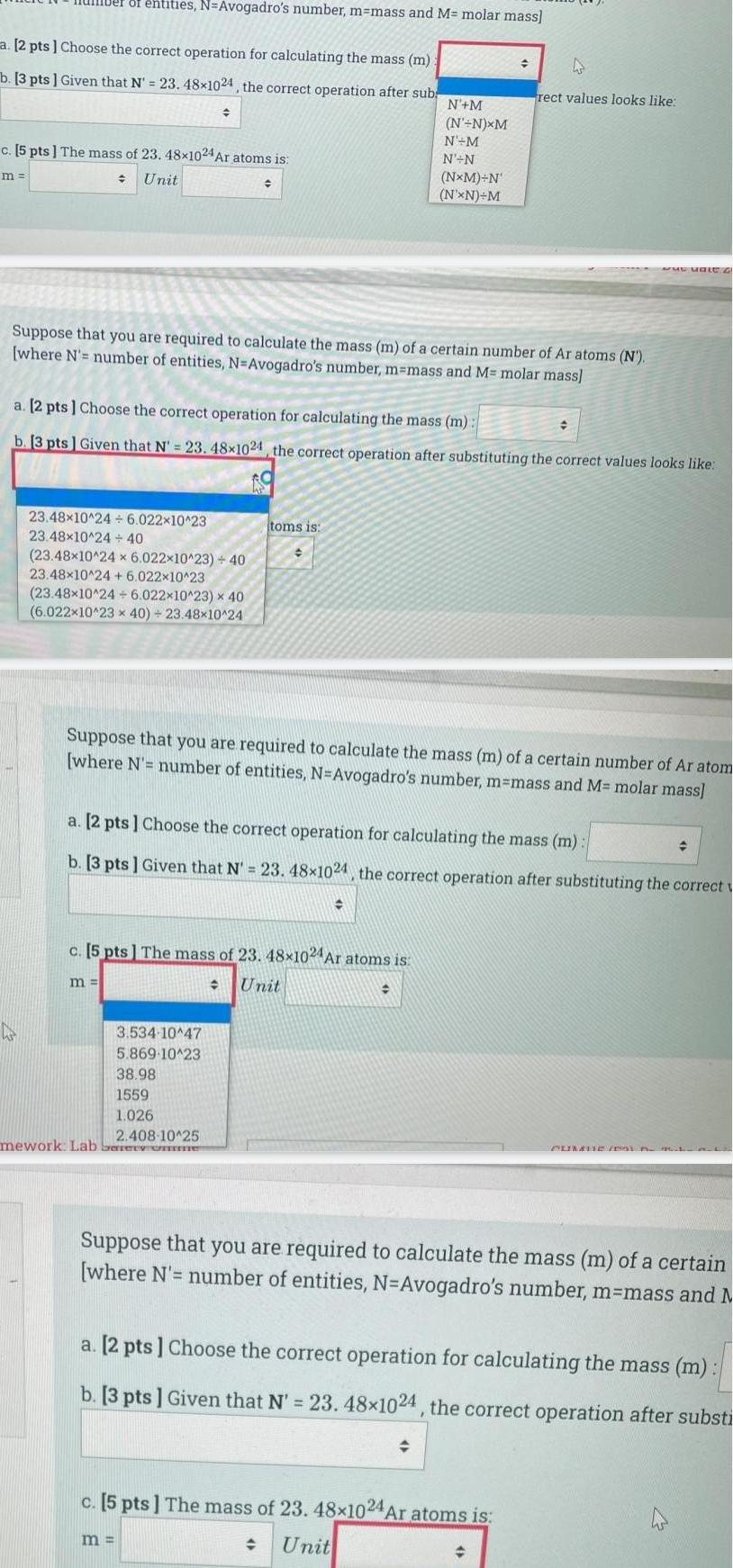
Generala 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48x1024 the correct operation after sub ber of entities N Avogadro s number m mass and M molar mass m c 5 pts The mass of 23 48x1024 Ar atoms is Unit 23 48x10 24 6 022 10 23 23 48x10 24 40 23 48x10 24 x 6 022x10 23 40 23 48x10 24 6 022x10 23 23 48x10 24 6 022x10 23 x 40 6 022x10 23 x 40 23 48x10 24 Suppose that you are required to calculate the mass m of a certain number of Ar atoms N where N number of entities N Avogadro s number m mass and M molar mass m a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substituting the correct values looks like 3 534 10 47 5 869 10 23 38 98 1559 1 026 2 408 10 25 mework Lab Sery one C h toms is m c 5 pts The mass of 23 48x1024 Ar atoms is Unit N M N N XM N M N N Suppose that you are required to calculate the mass m of a certain number of Ar atom where N number of entities N Avogadro s number m mass and M molar mass NxM N N XN M a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substituting the correct 4 rect values looks like c 5 pts The mass of 23 48x1024 Ar atoms is Unit www Male G Suppose that you are required to calculate the mass m of a certain where N number of entities N Avogadro s number m mass and M a 2 pts Choose the correct operation for calculating the mass m b 3 pts Given that N 23 48 1024 the correct operation after substi
Physical Chemistry
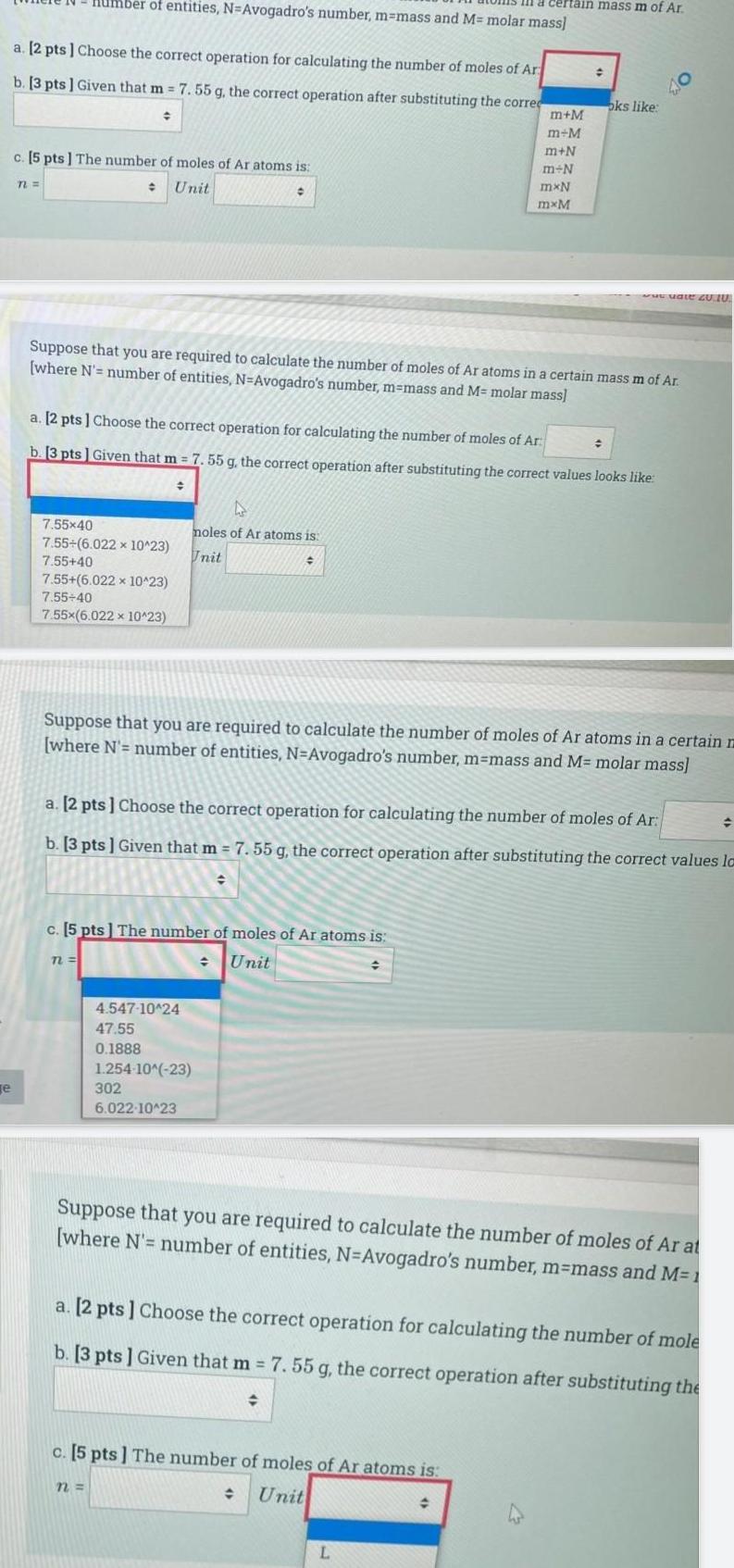
Generale a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correc c 5 pts The number of moles of Ar atoms is Unit n Imber of entities N Avogadro s number m mass and M molar mass 7 55x40 7 55 6 022 x 10 23 7 55 40 7 55 6 022 x 10 23 7 55 40 7 55x 6 022 x 10 23 72 Suppose that you are required to calculate the number of moles of Ar atoms in a certain mass m of Ar where N number of entities N Avogadro s number m mass and M molar mass a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correct values looks like c 5 pts The number of moles of Ar atoms is Unit D moles of Ar atoms is Unit 4 547 10 24 47 55 0 1888 n 1 254 10 23 302 6 022 10 23 Suppose that you are required to calculate the number of moles of Ar atoms in a certain m where N number of entities N Avogadro s number m mass and M molar mass certain mass m of Ar a 2 pts Choose the correct operation for calculating the number of moles of Ar b 3 pts Given that m 7 55 g the correct operation after substituting the correct values la m M m M m N m N mxN mxM c 5 pts The number of moles of Ar atoms is Unit oks like L 40 www uate 20 10 Suppose that you are required to calculate the number of moles of Ar at where N number of entities N Avogadro s number m mass and M 1 a 2 pts Choose the correct operation for calculating the number of mole b 3 pts Given that m 7 55 g the correct operation after substituting the

Physical Chemistry
SolutionsIn a certain titration experiment a student used 28 96 mL of an unknown NaOH solution to completely neutralize 25 00 mL of 0 1500 M HCl solution What is the molarity of the NaOH solution 1 0 1295 M O O 2 0 1738 M 3 7 723 M
Physical Chemistry
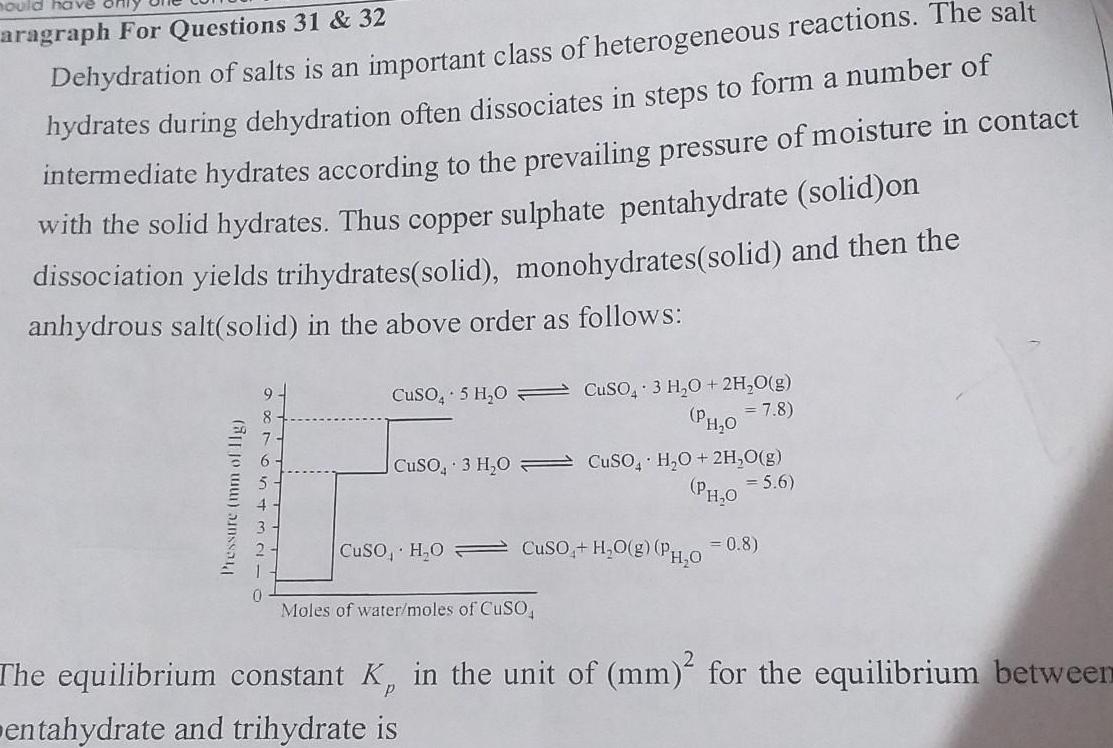
Generalhould have aragraph For Questions 31 32 Dehydration of salts is an important class of heterogeneous reactions The salt hydrates during dehydration often dissociates in steps to form a number of intermediate hydrates according to the prevailing pressure of moisture in contact with the solid hydrates Thus copper sulphate pentahydrate solid on dissociation yields trihydrates solid monohydrates solid and then the anhydrous salt solid in the above order as follows Pressure mm of lig CuSO4 5 H O CuSO3 H O SO4 H O 2H O g PH 0 5 6 CuSO H O CuSO4 H O g PH O 0 8 CuSO4 3 H O 2H O g PH 0 7 8 Moles of water moles of CuSO The equilibrium constant K in the unit of mm for the equilibrium between entahydrate and trihydrate is

Physical Chemistry
General3 pts each The atom with Z 16 a The atomic symbol is b The name of this atom is c This atom is in group d This atom is in period e This atom is classified as f The predicted ionic charge is 2 Ag sodium 6A 16 Na 2 iodine metalloid 2A 2 Ba nonmetal carbon barium C 5 S 1 silver 3 sulfur metal main gro 7A 17 22 27 37

Physical Chemistry
Atomic Structure1 d Find the de Broglie wavelength of the electrons that have been accelerated from rest through a potential difference of 40 kV Given electronic charge e 1 602x10 19 C me 9 109x10 31 kg 3

Physical Chemistry
General3 04 A solution containing 4 M Cr O 2 M Cr shows a pH of 2 0 Calculate reduction potential 2 303 RT F of half cell reaction 0 06 E Cr 0 Cr 1 33 V A 0 5 V B 0 7 V C 1 05 V D 1 50 V

Physical Chemistry
GeneralHow to find atomic mass of an elemen t I know you will say Number of proton s Number of neutrons for example ox ygen atomic 16 8 8 Number of pro tons Number of neutrons but how the chlorine have mass of 35 5 amu I know also this very well Because of the average mass of their isotopes b ut I want to know that oxygen also have isotopes but its atomic mass Number of protons Number of neutro ns why in the case of oxygen we don t c alculate the average of the mass of thei risotopes

Physical Chemistry
EquilibriumA chemical industry generates wastewater that contains ethanol 130 mg L and methanoic or formic acid 400 mg L If the discharge flow is 80 L s what will be the BOD of the wastewater due to these two substances B water contains a concentration of sulfate ions of the order of 1 0 x 10 3 M and of carbonate ions of 1 0 x 10 5 M It is desired to precipitate the sulfate as strontium sulfate and for this SrCl2 0 is used 1 mol dm3 a Will this procedure work Prove with calculations Data Kps 5 6 x 10 10 for strontium carbonate and 3 4 x 10 7 for strontium sulfate

Physical Chemistry
Solid stateAn element density 6 8 g cm3 occurs in bcc structure with cell edge of 290 pm Calculate the number of atoms present in 200 g of the element A B 24 12 x 1023 5 05 1021

Physical Chemistry
EquilibriumIn an aqueous solution of volume 500 ml when the reaction 2Ag Cu Cu 2Ag reaches equilibrium the concentration of Cu ions is x molar To this solution 500 ml of water is added At the new equilibrium the concentration of Cu ion would be A 2x molar 1 C less than x molar 2 B between x and 2x molar D between 0 005x molar and 0 5x molar

Physical Chemistry
Solid stateWBSU Year 2011 1 a What do you mean by Compton effect A beam of X rays is scattered by loosely bound electrons of a target through 45 to the direction of the beam What is the amount of Compton shift rest mass of the electron 9 109 10 31 kg h 6 625x10 34 J s 4

Physical Chemistry
Nuclear chemistry5 pts For 226Rn number of protons 5 pts For 222Rn number of protons th 5 pts For 226Rn number of protons 5 pts For 222Rn number of protons 222 86 45 30 136 5 pts For 226Rn number of protons 30 222 136 45 222Rn number of protons number of neutrons number of neutrons number of neutrons number of neutrons 226 45 140 222 136 number of neutrons number of neutrons and number of electrons and number of electrons gmatill I Due date 20 10 2 and number of electrons and number of electrons Next page Due date 20 and number of electrons and number of electrons

Physical Chemistry
Gaseous and liquid statesA cylinder containing a mixture of co an d co2 has a pressure of 2atm at 366k t he cylinder is cooled to 183k Where co s still a gas but co2 is a solid with a vap our pressure of 0 25 the pressure in cy inder at 183k is 0 90 what is the mole f raction of CO2 in cylinder

Physical Chemistry
Energetics03 A liquid confined inside an adiabatic container is suddenly taken from state 1 to state 2 by single stage process as shown then AH is A AH 2P Po 2yPo Vo 0 Y 1 C AH 3P V Vo 2 4V B AH PoVo 00 D AH 3yP Vo 0 0 V 1

Physical Chemistry
Generaldata Based on the R2 factor and the percent relative uncertainty for the data what correlation can you make If a correlation is determined discuss possible R2 factor 0 98346808 P R U of the slope x 100 1 69

Physical Chemistry
Chemical BondingConsider the resonance hybrid of the formate ion HCO shown below Which of the following best represents the charge of each oxygen in this structure 1 O 2 3 1 1 2 2 3 1 2 1 3 C 0 H C

Physical Chemistry
Equilibrium8 Calculate the ratio of pH of a solution containing 1 mole of CH COONa 1 mole of HCI per litre and of other solution containing 1 mole CH COONa 1mole of acetic acid per litre 1 1 1 2 2 1 3 1 2 4 2 3

Physical Chemistry
Electrochemistrywhile solving question in electrochemistry while finding electrode potential when do we use AG AG1 AG2 concept and when do we use E cell E cathode E anode please explain I will rate satisfied if I understand it 11 48

Physical Chemistry
Chemical kinetics9 LAW OF MASS ACTION In a chemical equilibrium the rate constant for the backward reaction is 7 5 x 104 and the equilibrium constant is 1 5 The rate constant for the forward reaction is 1 2x10 2 5 x 10 4 3 1 12 x 10 3 4 9 0 x 104 CAL EQUILIBRUM02EXEP65

Physical Chemistry
Atomic Structure2 d A monochromatic X ray beam whose wavelength is 0 0558 nm is scattered in Compton scattering through an angle 46 Calculate the wavelength of the scattered beam Given h 6 626x 10 34 J s mo rest mass of electron 9 1x10 31 kg c speed of light 2 998 108 m s 4

Physical Chemistry
Chemical kinetics48 If the rate of a reaction at 50 C is 2 6 x 10 mol L 5 then what will be rate of reaction at 80 C Given that the temperature coefficient is 3 1 7 02 x 10 2 7 025 x 10 3 7 8 x 10 4 none of these

Physical Chemistry
Equilibrium6 In a 0 25 L tube dissociation of 4 mol of NO takes place If its degree of dissociation is 10 The value of K for reaction 2 NO N O is 1 18 3 7 6 16 4 8 1 32 CROOCO 52

Physical Chemistry
Chemical kineticsChemical Kinetics 48 In three different reactions involving a single reactant in each case a plot of rate of the reaction on the y axis versus concentration of the reactant on the x axis gives three different curves shown below 0 0 The possible orders of the reactions i ii and iii respectively are 1 1 2 3 2 2 1 1 4 0 1 1 2 3 0 1 2 49 In which of the following option the example and the given order is incorrectly matched 1 H O H O 0 1st order 2 2NO Cl 2NOCI 2nd order i 3 CH COOCH NaOH CH COONa K A CH OH 2nd order p mm Hg 800 400 200 d B d C dt dt between K K and K is 2 0 5 4 2 2 1 5 4 3 5 52 For the reaction 2A B 3C if Telegram K A K A the correct relation 4 HI g H g 1 g Zero order 50 Consider the reaction 2x g 3y g 2z g At constant temperature and volume partial pressurece 220 s of x p varies as follows Time min 0 100 200 Order of the reaction is 1 O 3 1 51 The rate of reaction gets doubled when the temperature changes from 7 C to 17 C By what factor will it change for the temperature change from 17 C to 27 C assuming activation energy does not change with temperature 1 1 3 3 2 d A dt 53 Aakash Institute 1 K K K 2 2K K 3K 3 4K K 3K3 4 3K 6K 2K3 For a 1st order reaction if 180 minutes are required for completion of 99 9 of reaction time are required in minutes for completion of 90 of the reaction would be 1 30 2 10 4 80 3 60 54 Which of the following expression represents the time required for completion of th life of first order 4 reaction K 1 2 303 2 303 K 3 log4 55 For the first order reaction A K 2 2 303 1 2 7 3 25 57 If two first order reactions A B the reaction rate at 0 01 M concentration of A is found to be 2 0 x 10 5 M sec The half life period of the reaction is approximately 1 30 s log 2 303 K log3 If activation energy of a reaction is 800 cal mol at temperature 200 K The percentage of molecules having energy greater or equal to E is Given e 7 38 2 300 s 4 347 s KB 2 a second K 2 13 5 4 1 35 C D 2 b second If a b then the relation between K and K is 1 K K 2 K K 3 K K 4 K 2K 58 Consider the elementary reaction H g 2 g 2HI g If the volume of the container containing the gaseous mixture is increased to two times then rate of the reaction 1 Become four times 2 Become th of the original rate 3 Become 2 times

Physical Chemistry
GeneralThe temperature at which carbon reduced FeO into iron produces CO is 200 3 4 Correct Answer 4 Your Answer 3 4 Jour AG kJ 300 400 500 600 700 200 0 200 2Fe 0 2FeO Below temprature at point A Approx same temprature at point A Above than point A but below then point D temprature Above at point A temprature C 0 CO 20 0 200 400 800 1200 1600 2000 Temperature C POUR SELL UnationTZUEGUIUGIT rate 16 0

Physical Chemistry
EquilibriumQuestion 20 Given that at a certain temperature in a 1 5L vessel 5 0 mole of A 7 0 mole of B and 0 1 mole of C are present Then the value of equilibrium constant for the reaction A B 2C Heat is about a 7 22 x 10 5 b 2 31 10 4 c 7 22 10 4 d 6 11 x 105

Physical Chemistry
Chemical BondingThe magnetic moment of NiX41 ion is found to be zero Then the ion is X monodentate anionic ligand Question Type Single Correct Type 1 sp hybridized 2 spd2 hybridized

Physical Chemistry
Generalprovided as 37 Match the following Condition for the reaction to be favoured in forward direction COLUMN I A CO g H O 1 H CO aq AH 10kj mol B CO g 2H g CH OH g AH 91kj mol C N O g 2 NO g AH 57 2kJ mol D N g O g 2NO g AH 90 kJ mol A A p s B s C q r D q C A p B p s C q D q COLUMN II p Low temperature q High r s High B A p s B s C q r D q r D A p s B p s C q r D q temperature Low pressure pressure

Physical Chemistry
Equilibrium1 0 litre of solution which was in equilibrium with solid mixture of AgCl and Ag CrO was found to contain 1x 10 moles of Ag ions 1 0 x 10 moles of Cl ions and 8 0 x 10 moles of Cro ions Ag ions are added slowly to the above mixture keeping volume constant till 8 0 x 10 7 moles of AgCl got precipitated 3 Find the 4g left in the solution after precipitation of AgCl A 15 10 M B 5 10 M C 10 M D 10 M 4 How many moles of Ag CrO were precipitated A 7 68 x 10 moles C 3 20 x 10 moles ragraph For Questions 35 36 B 7 68 x 10 moles D 3 20 x 10 moles

Physical Chemistry
General16 2 mole of zinc is dissolved in HCl at 25 C The work done in open vessel is a 2 477 kJ c 0 0489 kJ b 4 955 kJ d None ogged by 2 C at constant TAL

Physical Chemistry
GeneralYour teacher has an addiction to Snickers bars food label below NUTRITION INFORMATION SERVINGS PER PACKAGE 1 SERVING SIZE 50g DI Per Average Quantities Per Serving Serve Per 100g ENERGY PROTEIN FAT Total Saturated CARBOHYDRATE Sugars SODIUM 1030kJ 12 4 4g 9 12 2g 17 4 5g 19 28 6g 9 25 6g 28 119mg 5 2020kJ 8 7g 24 0g 8 8g 56 5g 50 6g 236mg a Using the label and information provided how much energy in kJ is attributed to each of the major fo protein fats carbohydrates

Physical Chemistry
Chemical kinetics1 point Two chemicals A and B are combined to form a chemical C The rate of the reaction is proportional to the product of the instantaneous amounts of A and B not converted to chemical C Initially there are 64 grams of A and 59 grams of B and for each gram of B 1 6 grams of A is used It has been observed that 30 75 grams of C is formed in 5 minutes How much is formed in 30 minutes What is the limiting amount of C after a long time grams of C are formed in 30 minutes grams is the limiting amount of C after a long time

Physical Chemistry
Chemical kineticsreaction rate at 25 C 29 The following graph relates the initial concentration with Half life period for order reaction 1 Zero S 1 2 V conc 2 First 35

Physical Chemistry
SolutionsAt 300 K 36 g of glucose present in a litre of its solution has an osmotic pressure of 4 98 bar If the osmotic pressure of the solution is 1 52 bar at the same temperature what would be its concentration Report your answer upto two decimal places

Physical Chemistry
Chemical kinetics4 2 1 a 26 Select from the following that is an example of zero order reaction 1 2NH3 g PL1130K High pressure 2 H O H O g O g 3 C 2H22O11 H O 4 SnCl 2FeCl3 SnCl 2FeCl N g 3H g H C6H12O6 C6H12O6

Physical Chemistry
General9 The energy liberated when an excited electron returns to its ground state can have a any value from zero to infinity b only negative values c only specified positive values d none of the above ab 00

Physical Chemistry
EnergeticsMoving to the next ques on 7 Then a sample of aqueous hydrochloric acid was neutralized with aqueous sodium hydroxide in a calorimeter the temperature of 9 1 e reaction increased from 25 0 C to 31 5 C If the specific heat of water is 1 00 cal g C calculate the quantity of energy in calories involved in this eutralization reaction

Physical Chemistry
Energeticsg sample of NH4NO3 is heated in bomb calorimeter The temperature of the AT calorimeter increases by 6 32 K the heat capacity of the system is 1 23 kJ g deg The molar heat of decomposition for NH4NO3 is 1 16 1 kJ mol 3621 89 kJmol 2 7 53 kJmol 4 498 1 kJ mol

Physical Chemistry
Chemical kineticsConsider the elementary parallel reaction as given below B k A K C It is given that the half life period of the reactant A is 0 0693 min and at a giver instant the concentration of B is 3 times the concentration of C then the value of 10 k in min is

Physical Chemistry
EquilibriumIn a 1 0 L aqueous solution when the reaction 2 AB g Cu a Cu 2Ag reaches equilibrium Cu xM A4g M If volume of solution is doubled by adding water then at equilibrium A Cu M 48 MB Cu M 48 M 2 C Cu M 48 MC Cu M 48 M 2

Physical Chemistry
Atomic StructureLet s consider the case of nitrogen atoms which have the electronic configuration 18 28 2p in the ground state a Assume for now that the electrons are distinguishable particles so you don t have to worry about the symmetry of wavefunctions If we ignore the interaction between the electrons or spin orbit coupling effects what are all possible values for the total electronic quantum numbers S L and J of the highest occupied electronic shell in the ground state Here S is the total electronic spin L the total angular momentum and J the quantum number of J L S Use the spectroscopic notation 25 1L for all possible states b Now using the first Hund s rule and restoring indistinguishibility what is the total spin S of the highest occupied electronic shell in the ground state Is the spin part of the three electron ket state symmetric or antisymmetric under exchange What about the orbital part

Physical Chemistry
EquilibriumSolubility product of silver bromide is 5 0 x 10 13 The quantity of potassium bromide molar mass taken as 120 g mol to be added to 1 litre of 0 05 M solution of silver nitrate to start the precipitation of AgBr is AIEEE 2010 1 5 0 10 8 g 3 1 2 x 10 9 g 2 1 2 x 10 1 g 4 6 2 x 10 5 g

Physical Chemistry
General9 A solution contains Ag 0 14 Agtloin k ngta loilom KspAgI 8 5 10 17 M Ksp ng I 3 2 5 10 26 M Sri Chaitanya IIT Academy 35 27 12 15 Sr IPLCO JEE ADV 2014 P2 RPTA 16 Q Paper Calculate maximum concentration of iodide ion to be added from outsid which one of them gets precipitated almost completely A 8 5 10 MB 5 10 M C 1 7 x 10 M D 8 5 10 7 M What age of that metal ion is precipitated A 0 17 B 1 7 C 99 83 36 D 99 17

Physical Chemistry
General62 It s and S are solubility and solubility product of a sparingly soluble binary electrolyte respectively then 1 5 2 s 5 3 St JEO 20074 IN 200

Physical Chemistry
Chemical Bonding22 Fe 3 is more stable than Fe 2 the reason is are I 1st and 2nd I P difference is less than 11 0 eV III 2nd and 3rd IP difference is less than 11 0 eV The correct answer is 1 Only I 2 Only II II Core of Fe 3 is more stable IV IP of Fe 3 is high 3 I II and IV 4 II and I

Physical Chemistry
Surface chemistry1 3 and 4th point acche se samjha dijiye please or is question ka answer bhi bta dijiya 4 Which of the following is not correct 1 Activation of adsorbent increases the surface area for adsorption 2 Adsorption is surface phenomenon only 2 3 Adsorption of H and O on charcoal is chemical adsorption 4 Chemical adsorption is due to either covalent or ionic bonds

Physical Chemistry
Chemical kinetics5 For the elementary reaction MN the rate of disappearance of M increases by a factor of 8 upon doubling the concentration of M The order of the reaction with respect to M is b 3 d 1 a 4 c 2

Physical Chemistry
EnergeticsOut of the following properties how many are extensive properties 1 Resistance 2 E M F 3 lonic Mobility 4 Dipole Moment 5 Heat Capacity 6 Specific Heat 7 Densit 8 Specific volume A reversible reaction is carried out at 500k where its equilibrium constant in unity If AH at 500K is 4 K I The

Physical Chemistry
Electrochemistry81 A current of 2 0 A passed for 5 hours through a molten metal salt deposit of 22 2 g of metal Atomic weight 177 The oxidation state of the metal in the metal salt is a 1 b 2 c 3 d 4

Physical Chemistry
General50 Select the correct statement s 1 In Cr NH3 JCl3 the Werner primary valency is 3 ii Cr NH3 Cl3 is paramagnetic iii Co NH3 13 is a low spin complex 1 i ii and iii 3 ii and iii only 2 i and ii only 4 Only i