Understanding the arrangement of electrons in an atom is crucial in explaining its chemical behavior. Electron configuration describes how electrons are distributed among the atomic orbitals in an atom. By understanding the electron configuration, we can determine an atom’s valency, predict its properties, and interpret its atomic spectra.
What are Electron Configurations?
Electron configurations are a standard notation used to represent the distribution of electrons in an atom’s orbitals. Each electron-containing atomic subshell is placed in a sequence, with the number of electrons written in superscript. For example, the electron configuration of sodium is 1s2 2s2 2p6 3s1.
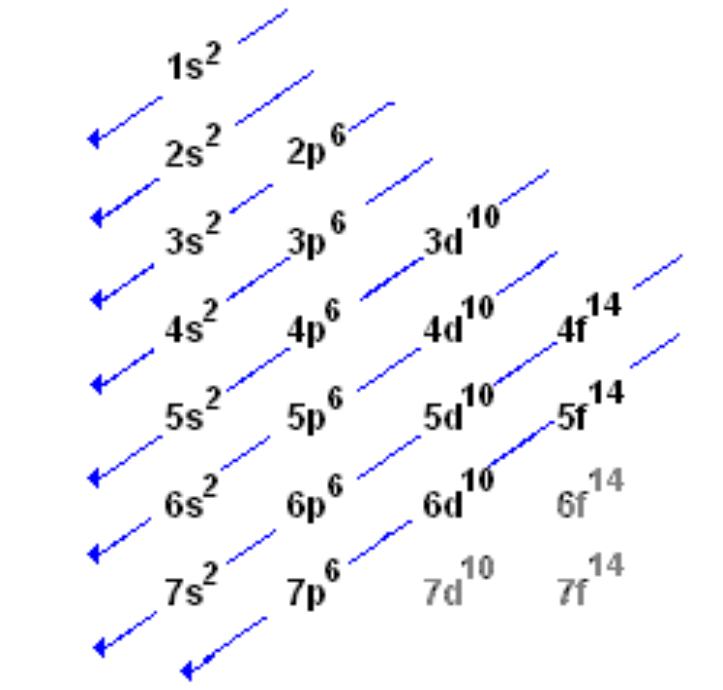
What are Atomic Orbitals?
Atomic orbitals are regions of space around the nucleus where electrons are likely to be found. These orbitals are characterized by different quantum numbers, such as the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). The combination of these quantum numbers gives rise to the unique properties of each atomic orbital.
Energy Quantization
According to the laws of quantum physics, electrons in an atom can only occupy certain energy levels or shells. The energy of an electron is quantized, meaning it can only exist in specific discrete energy states. The principal quantum number (n) determines the energy level of an electron, with higher values of n corresponding to higher energy levels.
Writing Electron Configurations
The electron configuration of an atom is represented using subshell labels and the number of electrons in each subshell. The subshell labels include the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number), and the total number of electrons in the subshell in superscript. For example, the electron configuration of magnesium (atomic number 12) is 1s2 2s2 2p6 3s2.
Shells
Shells are designated by the principal quantum number (n). The maximum number of electrons that can be accommodated in a shell is given by the formula 2n^2, where n is the shell number. For example, the first shell (n=1) can accommodate a maximum of 2 electrons, the second shell (n=2) can accommodate a maximum of 8 electrons, and so on.
Subshells
Subshells are designated by the azimuthal quantum number (l) and are based on the value of the principal quantum number (n). The subshells correspond to different orbital shapes and have different maximum electron capacities. The s subshell (l=0) can accommodate a maximum of 2 electrons, the p subshell (l=1) can accommodate a maximum of 6 electrons, the d subshell (l=2) can accommodate a maximum of 10 electrons, and the f subshell (l=3) can accommodate a maximum of 14 electrons.
Notation
The electron configuration of an atom is written using subshell labels. These labels include the shell number (given by the principal quantum number), the subshell name (given by the azimuthal quantum number), and the total number of electrons in the subshell in superscript. For example, if two electrons are filled in the ‘s’ subshell of the first shell, the resulting notation is ‘1s2’.
Filling of Atomic Orbitals
The filling of atomic orbitals follows certain principles, including the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
Aufbau Principle
The Aufbau principle states that electrons fill orbitals in order of increasing energy. Lower-energy orbitals are filled before higher-energy orbitals. This principle helps determine the order in which electrons occupy the atomic orbitals.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This means that each orbital can accommodate a maximum of two electrons with opposite spins.
Hund’s Rule
Hund’s rule states that electrons occupy orbitals of the same energy in a way that maximizes the total spin. This means that electrons will first fill separate orbitals within a subshell before pairing up in the same orbital.
Representation of Electronic Configuration of Atom
The electron configuration of an atom can be represented using subshell labels and the number of electrons in each subshell. This representation provides valuable information about an atom’s electron distribution and can be used to predict its chemical properties.
Electron Configuration of Hydrogen
Hydrogen, with an atomic number of 1, has one electron. This electron occupies the s subshell of the first shell. Therefore, the electron configuration of hydrogen is 1s1.
Electron Configuration of Oxygen
Oxygen, with an atomic number of 8, has eight electrons. These electrons are distributed in the following order: 1s2 2s2 2p4. The first shell (n=1) has two electrons in the s subshell, the second shell (n=2) has two electrons in the s subshell and four electrons in the p subshell.
Chlorine Electron Configuration
Chlorine, with an atomic number of 17, has 17 electrons. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5. The first shell (n=1) has two electrons in the s subshell, the second shell (n=2) has two electrons in the s subshell and six electrons in the p subshell, and the third shell (n=3) has two electrons in the s subshell and five electrons in the p subshell.
Uses of Electron Configuration
Electron configurations are useful in determining an element’s valency, predicting the properties of a group of elements, and interpreting atomic spectra. By understanding the electron configuration of an atom, we can gain insights into its chemical behavior and reactivity.
Electronic Configuration of First 30 Elements
The electron configurations of the first 30 elements are as follows:
| Atomic Number | Name of Elements | Electron Configuration |
|---|---|---|
| 1 | Hydrogen | 1s1 |
| 2 | Helium | 1s2 |
| 3 | Lithium | 1s2 2s1 |
| 4 | Beryllium | 1s2 2s2 |
| 5 | Boron | 1s2 2s2 2p1 |
| 6 | Carbon | 1s2 2s2 2p2 |
| 7 | Nitrogen | 1s2 2s2 2p3 |
| 8 | Oxygen | 1s2 2s2 2p4 |
| 9 | Fluorine | 1s2 2s2 2p5 |
| 10 | Neon | 1s2 2s2 2p6 |
| 11 | Sodium | 1s2 2s2 2p6 3s1 |
| 12 | Magnesium | 1s2 2s2 2p6 3s2 |
| 13 | Aluminum | 1s2 2s2 2p6 3s2 3p1 |
| 14 | Silicon | 1s2 2s2 2p6 3s2 3p2 |
| 15 | Phosphorus | 1s2 2s2 2p6 3s2 3p3 |
| 16 | Sulfur | 1s2 2s2 2p6 3s2 3p4 |
| 17 | Chlorine | 1s2 2s2 2p6 3s2 3p5 |
| 18 | Argon | 1s2 2s2 2p6 3s2 3p6 |
| 19 | Potassium | 1s2 2s2 2p6 3s2 3p6 4s1 |
| 20 | Calcium | 1s2 2s2 2p6 3s2 3p6 4s2 |
| 21 | Scandium | 1s2 2s2 2p6 3s2 3p6 4s2 3d1 |
| 22 | Titanium | 1s2 2s2 2p6 3s2 3p6 4s2 3d2 |
| 23 | Vanadium | 1s2 2s2 2p6 3s2 3p6 4s2 3d3 |
| 24 | Chromium | 1s2 2s2 2p6 3s2 3p6 4s1 3d5 |
| 25 | Manganese | 1s2 2s2 2p6 3s2 3p6 4s2 3d5 |
| 26 | Iron | 1s2 2s2 2p6 3s2 3p6 4s2 3d6 |
| 27 | Cobalt | 1s2 2s2 2p6 3s2 3p6 4s2 3d7 |
| 28 | Nickel | 1s2 2s2 2p6 3s2 3p6 4s2 3d8 |
| 29 | Copper | 1s2 2s2 2p6 3s2 3p6 4s1 3d10 |
| 30 | Zinc | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 |
Purpose of Electron Configurations
The purpose of electron configurations is to provide a systematic representation of how electrons are arranged in an atom. By understanding the electron configuration, we can determine an atom’s valency, predict its chemical properties, and interpret its atomic spectra. Electron configurations are a fundamental concept in chemistry and play a crucial role in understanding the behavior of atoms and molecules.
The Shielding Effect and Effective Nuclear Charge
The shielding effect refers to the reduction in attraction between an electron and the nucleus due to the presence of other electrons in the same atom. This shielding effect results in a decrease in the effective nuclear charge experienced by an electron. The effective nuclear charge is the net positive charge experienced by an electron and determines its energy level and stability. The shielding effect and effective nuclear charge influence the electron configuration and chemical properties of an atom.

Solved Examples on Electron Configuration
Let’s solve a few examples to understand how to write electron configurations for different elements.
Example 1: Write the electron configuration for chromium (Cr).
Solution: The atomic number of chromium is 24. Following the Aufbau principle, we fill the orbitals in order of increasing energy. The electron configuration of chromium is [Ar] 4s2 3d4.
Example 2: Write the electron configuration for sulfur (S).
Solution: The atomic number of sulfur is 16. Following the Aufbau principle, the electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4.
Example 3: Write the electron configuration for silicon (Si).
Solution: The atomic number of silicon is 14. Following the Aufbau principle, the electron configuration of silicon is 1s2 2s2 2p6 3s2 3p2.
How Kunduz Can Help You Learn Electron Configuration?
Kunduz is your trusted companion in learning electron configuration. Our expert team of chemistry teachers is dedicated to providing comprehensive and easy-to-understand explanations of electron configuration. We offer step-by-step guidance and examples to help you grasp the concept and apply it to various elements. Whether you are a student or a chemistry enthusiast, Kunduz is here to support your learning journey. With our user-friendly interface and interactive resources, you can master electron configuration and excel in your chemistry studies.
