In the realm of chemistry, we encounter various types of substances. Some substances are pure, while others are mixtures. Mixtures, in particular, are a combination of two or more pure substances. These substances can be elements or compounds that are mixed together without undergoing any chemical changes. One type of mixture that we come across frequently is a heterogeneous mixture.
What is a Heterogeneous Mixture?
A heterogeneous mixture is a type of mixture characterized by its non-uniform composition. In other words, the components of a heterogeneous mixture are not evenly distributed throughout, and they can be easily distinguished from one another. When we observe a heterogeneous mixture, we can see its individual components with the naked eye. These components can vary in terms of their physical and chemical properties.
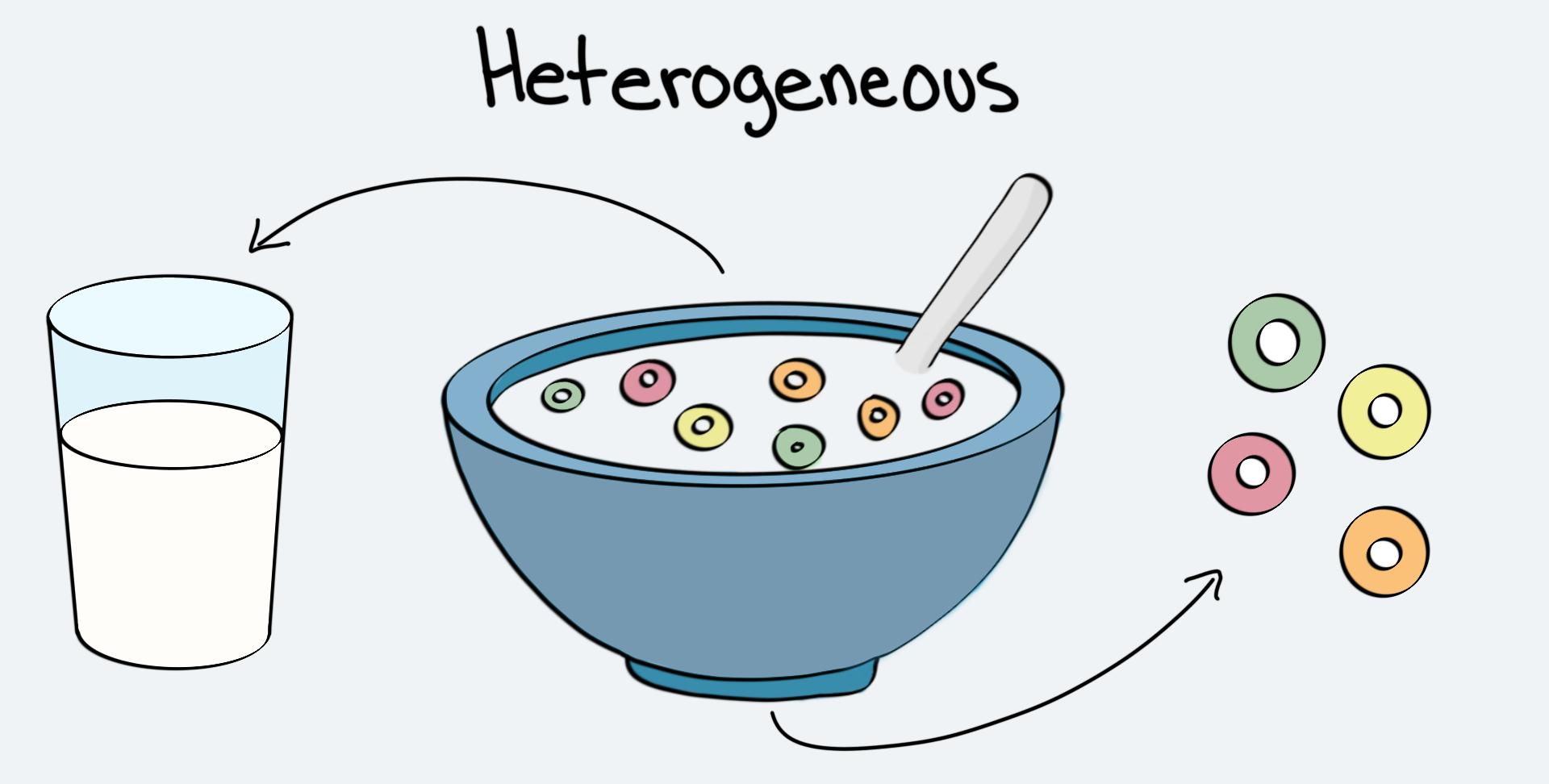
What is a Homogeneous Mixture?
Before delving deeper into heterogeneous mixtures, it’s important to understand the concept of a homogeneous mixture. Unlike heterogeneous mixtures, homogeneous mixtures have a uniform composition, meaning that their components are evenly distributed throughout the mixture. Homogeneous mixtures are also referred to as solutions. In a homogeneous mixture, the individual components cannot be easily distinguished.
To illustrate the difference between homogeneous and heterogeneous mixtures, let’s consider an example. If we mix salt in water and the salt completely dissolves, we have a homogeneous mixture. On the other hand, if we mix sand in water, the sand particles will not dissolve and will remain visible, resulting in a heterogeneous mixture.
Differences between Heterogeneous vs. Homogeneous Mixtures
To better understand the characteristics and properties of heterogeneous mixtures, let’s compare them to homogeneous mixtures in a detailed table:
| Feature | Heterogeneous Mixtures | Homogeneous Mixtures |
|---|---|---|
| Composition | Components are not evenly distributed | Components are evenly distributed |
| Visibility | Components can be easily distinguished | Components cannot be easily distinguished |
| Examples | Salad, soil, granite | Saltwater, air, vinegar |
| Separation Methods | Filtration, centrifugation, decantation | Evaporation, distillation, chromatography |
| Physical Properties | Properties can vary across different regions of mixtures | Properties are consistent throughout the mixture |

Composition of Heterogeneous Mixtures
Heterogeneous mixtures can be composed of a wide variety of substances. These mixtures can consist of solids, liquids, or gases. When different substances are combined to form a heterogeneous mixture, they may not blend completely, resulting in a non-uniform distribution of components.
For example, soil is a common example of a heterogeneous mixture. It is composed of various components such as minerals, organic matter, water, and air. The composition of soil can vary depending on its location and the specific conditions in that area.
Characteristics of Heterogeneous Mixtures
Heterogeneous mixtures possess several key characteristics that distinguish them from other types of mixtures. These characteristics include:
No Physical Change: Heterogeneous mixtures consist of components that do not undergo any chemical changes when mixed together. The individual components retain their own chemical properties.
Variable Composition: The components of a heterogeneous mixture can vary in their proportions. Unlike compounds, which have fixed ratios of elements, mixtures can have different ratios depending on the specific mixture.
No Specific Physical Properties: Heterogeneous mixtures do not have fixed melting or boiling points. The properties of a heterogeneous mixture are an average of the properties of its individual components.
Homogeneity: Unlike homogeneous mixtures, which have a uniform composition, heterogeneous mixtures have a non-uniform composition. The components of a heterogeneous mixture can be visually distinguished from one another.
Separation: Components of a heterogeneous mixture can be separated using various physical methods. These methods include filtration, centrifugation, decantation, and evaporation.
Energy Change: The formation of a heterogeneous mixture does not usually involve any energy changes, such as the release or absorption of heat or light.
Properties of Heterogeneous Mixtures
Heterogeneous mixtures exhibit several properties that are distinct from homogeneous mixtures. These properties include:
Particle Size: The particles in a heterogeneous mixture can vary in size. Some particles may be large and visible, while others may be smaller and not easily distinguishable.
Settling: In some heterogeneous mixtures, the larger particles may settle at the bottom over time due to gravity. This settling can result in a visible separation of components.
Scattering of Light: Heterogeneous mixtures can scatter light, making them appear cloudy or opaque. This phenomenon is known as the Tyndall effect. The scattering of light occurs when the particles in the mixture are large enough to interact with and deflect the light.
Types of Heterogeneous Mixtures
Heterogeneous mixtures can be further categorized into different types based on their physical properties and composition. The main types of heterogeneous mixtures include:
Suspensions
A suspension is a type of heterogeneous mixture where solid particles are dispersed in a liquid or gas medium. The solid particles in a suspension are typically larger and can settle over time. Examples of suspensions include muddy water, sand in water, and paint.
Colloids
Colloids are another type of heterogeneous mixture where small particles are dispersed in a medium. Unlike suspensions, the particles in colloids are smaller and do not settle. Colloids can exhibit unique properties such as the Tyndall effect and can be categorized into different types based on the states of the dispersed phase and the dispersion medium. Examples of colloids include milk, fog, and gelatin.
Solids
Heterogeneous mixtures can also exist in the solid state. In these mixtures, different solid substances are mixed together, resulting in a non-uniform distribution. Examples of solid heterogeneous mixtures include soil, granite, and mixed nuts.
Liquids
Liquid heterogeneous mixtures consist of different liquid substances that are not completely soluble in each other. The liquids do not form a single phase and can be visually distinguished. An example of a liquid heterogeneous mixture is oil and water.
Gases
While gases are typically homogeneous in nature, certain mixtures can be considered heterogeneous when different gases are not uniformly distributed. Examples include smog, which is a mixture of smoke and fog, and air pollution caused by the presence of various gases and particulate matter.

Division of Mixtures Methods
To separate the components of a heterogeneous mixture, various methods can be employed. These methods take advantage of the physical properties and differences of the individual components. Some common methods of separating mixtures include:
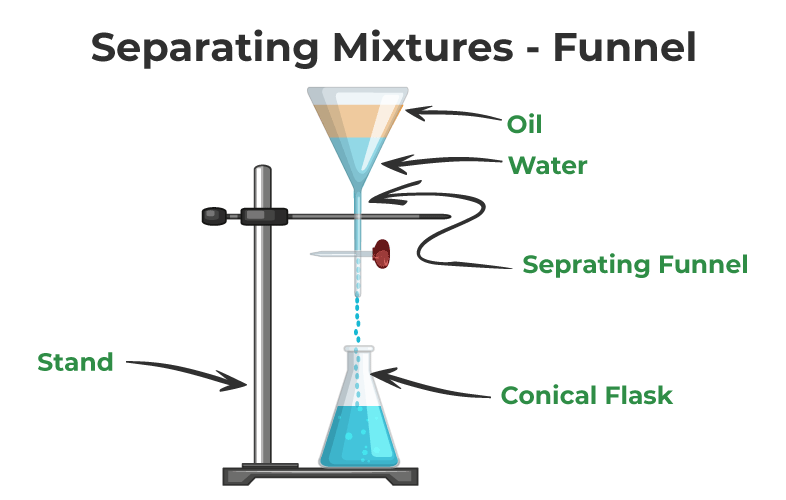
Filtration
Filtration is a commonly used method to separate solid particles from a liquid or gas medium. It involves passing the mixture through a filter or porous material that allows the liquid or gas to pass through while retaining the solid particles.
Centrifugation
Centrifugation is a technique that utilizes centrifugal force to separate components of a mixture based on their density. The mixture is placed in a rotating container, and the centrifugal force causes the denser components to move towards the outer edge, allowing for separation.
Decantation
Decantation is the process of carefully pouring off a liquid from a mixture, leaving the solid particles behind. This method is typically used when the solid particles have settled at the bottom of the container.
Evaporation
Evaporation is a technique that involves heating a mixture to vaporize the liquid component, leaving behind the solid particles. The vaporized liquid can then be condensed and collected separately.
Chromatography
Chromatography is a separation technique that utilizes the differential affinity of components in a mixture for a stationary phase and a mobile phase. The mixture is passed through a medium, and the components separate based on their different rates of movement.
Fractional Distillation
Fractional distillation is a method used to separate a mixture of liquids with different boiling points. The mixture is heated, and the components vaporize at different temperatures. The vapor is then condensed and collected separately.
How to Identify Heterogeneous Mixtures?
Identifying a heterogeneous mixture can be done by visually examining the mixture. Look for visible differences in color, texture, or particle size. If you can see distinct components within the mixture that do not blend together uniformly, it is likely a heterogeneous mixture.
Heterogeneous Mixtures Examples
Heterogeneous mixtures can be found in various aspects of our daily lives. Here are some common examples of heterogeneous mixtures:
- Salad: A salad is a mixture of various ingredients such as lettuce, tomatoes, cucumbers, and dressing. These ingredients can be visually distinguished and do not blend together uniformly.
- Soil: Soil is a complex mixture of minerals, organic matter, water, and air. Its composition can vary depending on the location and environmental conditions.
- Granite: Granite is a natural rock composed of different minerals, giving it a speckled appearance.
- Muddy Water: Muddy water is a suspension where soil particles are dispersed in water. The particles can settle over time, resulting in a non-uniform distribution.
Frequently Asked Questions on Heterogeneous Mixtures
In this section, let’s tackle some common queries regarding heterogeneous mixtures:
What is a heterogeneous mixture?
A heterogeneous mixture is a concoction where the components are not uniformly distributed, leading to various discernible phases within the mixture.
Is soil a heterogeneous mixture?
Yes, soil is a heterogeneous mixture. It is composed of various substances like organic matter, minerals, water, and living organisms.
Is air heterogeneous or homogeneous?
Pure air is considered a homogeneous mixture. However, when pollutants like dust and smoke are present, air becomes a heterogeneous mixture.
Is milk homogeneous or heterogeneous?
Milk is considered a colloid, a type of heterogeneous mixture. The fat droplets in milk are dispersed throughout the liquid, but they remain separate.
Is salt water homogeneous or heterogeneous?
Saltwater is a homogeneous mixture. The salt is evenly distributed in the water, creating a uniform solution.
Is sugar water homogeneous or heterogeneous?
Sugar water is a homogeneous mixture. The sugar, once dissolved, is evenly distributed throughout the water.
How Kunduz Can Help You Learn Heterogeneous Mixtures?
At Kunduz, we understand the importance of learning and grasping complex scientific concepts like heterogeneous mixtures. Whether you need assistance with theoretical concepts or practical applications, our tutors are here to guide you every step of the way. Don’t let the complexities of chemistry hold you back. Join Kunduz and embark on your journey to academic success today!
