The Law of Conservation of Mass is a fundamental principle in physics and chemistry that states that the mass of a closed system remains constant over time. This means that mass cannot be created or destroyed in a chemical reaction, but can only be transformed from one form to another. The concept of mass conservation is essential in various fields, including mechanics, thermodynamics, and fluid dynamics.
Understanding the Law of Conservation of Mass is crucial in comprehending the behavior of matter and energy in different systems. It allows scientists and engineers to predict and analyze the outcome of chemical reactions and physical processes. In this article, we will delve into the definition, formula, examples, and applications of the Law of Conservation of Mass.
What is the Law of Conservation of Mass?
The Law of Conservation of Mass, also known as the Law of Conservation of Matter, states that the total mass of a closed system remains constant before and after a chemical reaction. This means that the mass of the reactants must be equal to the mass of the products. In other words, the atoms of the reactants are rearranged to form new compounds, but the total number of atoms and their mass remains unchanged.
The law is based on the assumption that atoms are indivisible and cannot be created or destroyed. It was first formulated by Antoine Lavoisier, a French chemist, in the late 18th century. Lavoisier’s experiments demonstrated that the total mass of substances involved in a chemical reaction remains constant, even if the substances undergo physical or chemical changes.
The Law of Conservation of Mass is a fundamental principle in chemistry and plays a crucial role in stoichiometry, the calculation of the quantities of reactants and products in a chemical reaction. It allows chemists to determine the mass of reactants required to produce a certain amount of product and vice versa.
Formula of the Law of Conservation of Mass
The Law of Conservation of Mass can be expressed mathematically using the formula:
Mass of Reactants = Mass of Products
This formula states that the mass of the reactants before a chemical reaction is equal to the mass of the products after the reaction. The equation holds true for all chemical reactions, regardless of the complexity or number of substances involved.
In fluid mechanics and continuum mechanics, the law of conservation of mass is expressed in the continuity equation. The continuity equation represents the conservation of mass on a local level and is given by:
∂ρ/∂t + ∇⋅(ρv) = 0
Where:
- ∂ρ/∂t is the rate of change of density with respect to time
- ∇⋅(ρv) is the divergence of the mass flux density vector, with ρ representing density and v representing velocity
This equation states that the change in density over time, combined with the divergence of the mass flux density, must be zero in a closed system.
Law of Conservation of Mass
The Law of Conservation of Mass is a fundamental principle in physics and chemistry that has far-reaching implications. It states that in a closed system, the total mass remains constant over time. This means that mass cannot be created or destroyed, but only transformed from one form to another.
The law is based on the assumption that atoms are indivisible and that no mass is lost or gained during a chemical reaction. It applies to all chemical reactions, from simple reactions involving two substances to complex reactions involving multiple reactants and products.
The conservation of mass allows scientists and engineers to predict and analyze the outcome of chemical reactions. By understanding the Law of Conservation of Mass, chemists can determine the quantities of reactants needed to produce a certain amount of product or calculate the mass of products obtained from a given amount of reactants.
The Law of Conservation of Mass is closely related to the Law of Conservation of Energy, which states that energy cannot be created or destroyed, only transformed from one form to another. The two laws are interconnected through the concept of mass-energy equivalence, as described by Albert Einstein’s famous equation E=mc².
In summary, the Law of Conservation of Mass is a fundamental principle that governs the behavior of matter in chemical reactions. It ensures that the total mass of a closed system remains constant, allowing scientists to make accurate predictions and calculations in various scientific fields.
Law of Conservation of Mass Problems
The Law of Conservation of Mass is a powerful tool for solving problems in chemistry and physics. By applying the principle that mass is conserved in a closed system, we can determine the quantities of reactants and products in a chemical reaction.
Let’s solve a problem to illustrate how the Law of Conservation of Mass is applied:
Problem: 10 grams of calcium carbonate (CaCO3) produces 3.8 grams of carbon dioxide (CO2) and 6.2 grams of calcium oxide (CaO). Show that these observations are in agreement with the law of conservation of mass.
Solution: According to the Law of Conservation of Mass, the mass of the reactants must be equal to the mass of the products. Let’s calculate the total mass of the reactants and products in this reaction:
Mass of reactants = Mass of products 10 grams of CaCO3 = 3.8 grams of CO2 + 6.2 grams of CaO
As we can see, the total mass of the reactants (10 grams) is equal to the total mass of the products (3.8 grams + 6.2 grams = 10 grams). Therefore, these observations are in agreement with the Law of Conservation of Mass.
By solving similar problems, we can verify that mass is conserved in chemical reactions and gain a deeper understanding of the Law of Conservation of Mass.
Limitation of the Law of Conservation of Mass
While the Law of Conservation of Mass is a fundamental principle in physics and chemistry, it has certain limitations. These limitations arise in situations where nuclear reactions or particle-antiparticle annihilation occur, as these processes involve a conversion of mass into energy.
In nuclear reactions, such as nuclear fission or fusion, a small amount of mass is converted into energy according to Einstein’s famous equation E=mc². This equation shows the equivalence between mass and energy, stating that mass can be converted into energy and vice versa.
Similarly, in particle-antiparticle annihilation, a particle and its corresponding antiparticle collide and annihilate each other, converting their mass into energy.
In these cases, the total mass of the system is not conserved, as some of the mass is transformed into energy. However, the total energy, including both mass and other forms of energy like kinetic energy and potential energy, is conserved.
It is important to note that these exceptions to the Law of Conservation of Mass do not invalidate the principle in most everyday chemical reactions. The conversion of mass into energy is significant only in highly energetic systems, such as nuclear reactions or particle physics experiments.
In summary, while the Law of Conservation of Mass is a fundamental principle, it has limitations in situations involving nuclear reactions or particle-antiparticle annihilation, where mass can be converted into energy.
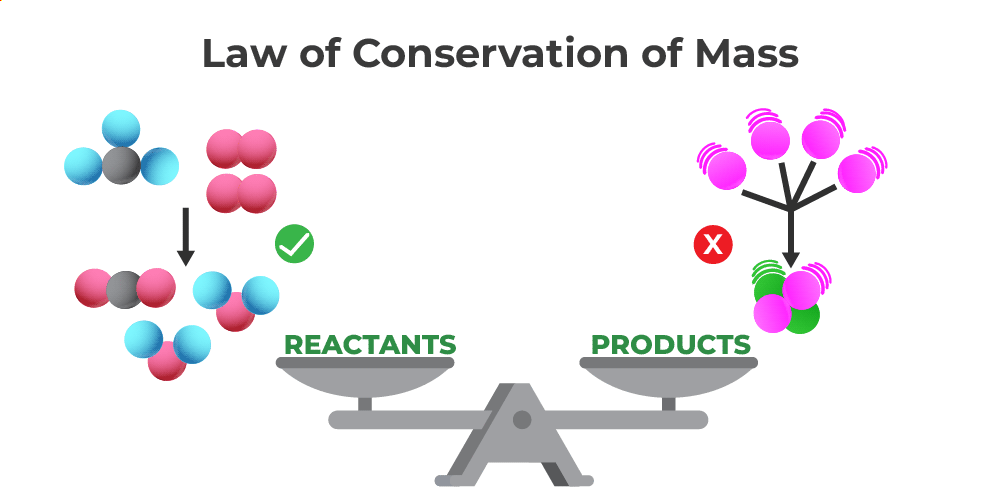
Law of Conservation of Matter
The Law of Conservation of Matter is another term used to refer to the Law of Conservation of Mass. Both terms describe the same fundamental principle that states that matter cannot be created or destroyed in a chemical reaction, but only transformed from one form to another.
The Law of Conservation of Matter is often used interchangeably with the Law of Conservation of Mass, especially in the context of chemical reactions and stoichiometry. Both terms highlight the preservation of matter in a closed system, ensuring that the total mass of the reactants is equal to the total mass of the products.
It is important to note that the term “matter” refers to the substance or material that makes up physical objects. Matter is composed of atoms and molecules, which are indivisible and conserved in a chemical reaction.
The Law of Conservation of Matter, like the Law of Conservation of Mass, plays a crucial role in chemistry and allows scientists to predict and analyze the outcome of chemical reactions. By understanding the preservation of matter, chemists can calculate the quantities of reactants and products, determine the stoichiometry of a reaction, and make accurate predictions about the behavior of substances.
In conclusion, the Law of Conservation of Matter is a fundamental principle that describes the preservation of matter in a chemical reaction. It emphasizes that matter cannot be created or destroyed but only transformed, ensuring the total mass of a closed system remains constant.
Life and the Law of Conservation of Mass
The Law of Conservation of Mass is not only applicable in the realm of physics and chemistry but also plays a significant role in understanding the dynamics of life and the environment. The preservation of mass is crucial for the functioning of living organisms and the balance of ecosystems.
In living organisms, the Law of Conservation of Mass ensures that matter is conserved during various biological processes, such as metabolism, growth, and reproduction. The atoms and molecules that make up living organisms are constantly being rearranged and transformed, but the total mass remains constant.
For example, when plants undergo photosynthesis, they convert carbon dioxide (CO2) and water (H2O) into glucose (C6H12O6) and oxygen (O2). The mass of the reactants (CO2 and H2O) is equal to the mass of the products (glucose and O2), adhering to the Law of Conservation of Mass.
Similarly, in the human body, the Law of Conservation of Mass ensures that the mass of the food we consume is equal to the mass of the waste products we excrete. The nutrients from food are broken down and transformed into energy, tissues, and waste products, but the total mass remains constant.
On a larger scale, the Law of Conservation of Mass plays a crucial role in understanding the balance of ecosystems. The exchange of matter between organisms and their environment is governed by the conservation of mass. Nutrients are cycled through various organisms in a delicate balance, ensuring the sustainability of ecosystems.
Understanding the Law of Conservation of Mass allows scientists and ecologists to study and predict the flow of matter in ecosystems, as well as the impact of human activities on the environment. It emphasizes the importance of sustainable practices and the need to minimize waste and pollution.
In summary, the Law of Conservation of Mass is not limited to the realm of physics and chemistry but also applies to the dynamics of life and ecosystems. It ensures the preservation of matter in biological processes and guides our understanding of the balance of nature.
Mass Balance of Elements in Organisms
The Law of Conservation of Mass is essential for understanding the mass balance of elements in living organisms. Elements, such as carbon, nitrogen, and oxygen, are cycled through various biological processes, ensuring the conservation of mass in organisms.
In the biosphere, elements are constantly being exchanged between organisms, the atmosphere, the hydrosphere, and the lithosphere. This exchange is driven by biological processes, such as photosynthesis, respiration, decomposition, and nutrient uptake.
Photosynthesis is a key process that converts carbon dioxide from the atmosphere into organic compounds, such as glucose, in plants. During photosynthesis, the carbon atoms from carbon dioxide are incorporated into organic molecules, while oxygen is released as a byproduct. This process allows plants to store energy in the form of chemical bonds and contributes to the mass balance of carbon in ecosystems.
Respiration, on the other hand, is a process that occurs in all living organisms, including plants, animals, and microorganisms. During respiration, organic compounds, such as glucose, are broken down to release energy, carbon dioxide, and water. The carbon dioxide released during respiration is returned to the atmosphere, completing the carbon cycle.
Decomposition, carried out by decomposers such as bacteria and fungi, plays a crucial role in the recycling of nutrients in ecosystems. Decomposers break down dead organic matter, releasing nutrients back into the soil or water. This process ensures the mass balance of elements, such as nitrogen and phosphorus, which are essential for plant growth.
Nutrient uptake by plants and animals also contributes to the mass balance of elements in organisms. Plants absorb nutrients from the soil, while animals obtain nutrients by consuming plants or other animals. The nutrients are incorporated into the tissues of organisms and are eventually returned to the environment through excretion or decomposition.
The Law of Conservation of Mass governs the mass balance of elements in organisms. It ensures that the total mass of elements remains constant in biological processes, allowing for the sustainability of ecosystems. Understanding the mass balance of elements is crucial for studying nutrient cycling, ecological interactions, and the impact of human activities on the environment.
Mass Balance in Watersheds
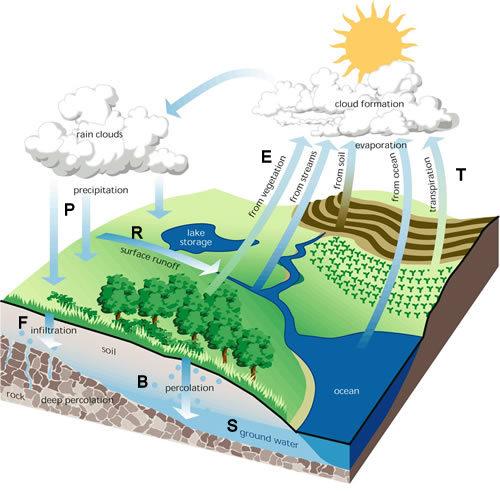
The Law of Conservation of Mass is vital for studying the mass balance in watersheds, which are the drainage basins of rivers and streams. Watersheds are dynamic systems where water and sediment are transported through various processes, influencing the mass balance of elements and nutrients.
In a watershed, precipitation falls onto the land surface and is either infiltrated into the soil or runs off into streams and rivers. This water movement plays a crucial role in the transport of nutrients, sediments, and pollutants.
The Law of Conservation of Mass ensures that the total mass of water and sediment entering a watershed is equal to the total mass leaving the watershed. This principle allows scientists to study the movement of water and sediment, as well as the distribution of nutrients and pollutants, in watersheds.
Nutrients, such as nitrogen and phosphorus, are essential for plant growth but can become pollutants when present in excessive amounts. Understanding the mass balance of nutrients in watersheds is crucial for managing water quality and preventing eutrophication, a process characterized by excess nutrient enrichment and algal blooms.
Sediment transport is another important aspect of the mass balance in watersheds. Sediment, including soil particles and organic matter, is eroded from the land surface by rainfall and runoff. It is then transported by streams and rivers, eventually settling in lakes, reservoirs, or the ocean. The Law of Conservation of Mass ensures that the total mass of sediment eroded from the land is equal to the total mass deposited in aquatic systems.
By studying the mass balance in watersheds, scientists can assess the impact of land use changes, such as deforestation or urbanization, on water quality and sediment transport. They can also develop strategies for managing water resources and mitigating the effects of pollution and erosion.
In summary, the Law of Conservation of Mass is crucial for understanding the mass balance in watersheds. It ensures that the total mass of water, sediment, and nutrients entering and leaving a watershed remains constant, allowing scientists to study the movement of water and sediment and manage water resources effectively.
Mass Balance in Human-Dominated Ecosystems
Human activities have a significant impact on the mass balance in ecosystems, particularly in human-dominated ecosystems such as urban areas and agricultural landscapes. Understanding the mass balance in these ecosystems is essential for sustainable management and resource allocation.
In urban areas, human activities can lead to the accumulation of waste and pollutants, which can disrupt the mass balance of elements and nutrients. Improper waste management can result in the release of contaminants into the environment, affecting water quality, soil fertility, and biodiversity.
The Law of Conservation of Mass ensures that the total mass of waste generated in urban areas is equal to the total mass of waste managed or disposed of. By implementing effective waste management strategies, such as recycling, composting, and proper disposal, the mass balance in urban ecosystems can be maintained.
Agricultural landscapes also require careful management to ensure the mass balance of nutrients and prevent soil degradation. Agricultural practices, such as the use of fertilizers and irrigation, can lead to the accumulation of nutrients in soils and water bodies. This can result in eutrophication, decreased water quality, and loss of biodiversity.
The Law of Conservation of Mass is essential for managing nutrient cycling in agricultural systems. By optimizing fertilizer use, practicing crop rotation, and implementing conservation practices, farmers can maintain the mass balance of nutrients and minimize the environmental impact of agriculture.
In addition to waste management and agriculture, the mass balance in human-dominated ecosystems is influenced by other factors, such as energy consumption, transportation, and land use changes. By considering the principles of mass conservation, policymakers and planners can make informed decisions to ensure sustainable development and resource management.
In conclusion, the Law of Conservation of Mass is applicable in human-dominated ecosystems, where human activities can disrupt the mass balance of elements and nutrients. By understanding and managing the mass balance in these ecosystems, we can promote environmental sustainability and ensure the long-term well-being of both human and natural systems.
Law of Conservation of Mass in Chemistry
The Law of Conservation of Mass is of utmost importance in chemistry, where it serves as a fundamental principle for understanding and predicting the outcome of chemical reactions. By applying the law, chemists can determine the quantities of reactants and products involved in a reaction.
Chemical reactions involve the rearrangement of atoms to form new substances. According to the Law of Conservation of Mass, the total mass of the reactants must be equal to the total mass of the products. This means that atoms are neither created nor destroyed during a chemical reaction, but are only rearranged.
Chemists express the Law of Conservation of Mass using the formula:
Mass of Reactants = Mass of Products
This equation allows chemists to balance chemical equations by ensuring that the number of atoms on both sides of the equation is equal. Each element must have the same number of atoms before and after the reaction, maintaining the conservation of mass.
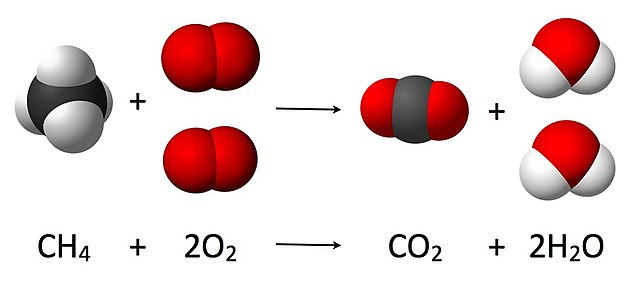
For example, let’s consider the combustion of methane (CH4) in the presence of oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). The balanced chemical equation for this reaction is:
CH4 + 2O2 → CO2 + 2H2O
By balancing the equation, we ensure that the number of carbon, hydrogen, and oxygen atoms is the same on both sides of the equation, thereby conserving mass.
The Law of Conservation of Mass allows chemists to calculate the quantities of reactants required to produce a certain amount of product, or vice versa. This is crucial for industrial processes, where the efficient use of resources is essential.
In addition to balancing chemical equations, the Law of Conservation of Mass is also applied in stoichiometry, the calculation of the quantities of reactants and products in a chemical reaction. Stoichiometry allows chemists to determine the mole ratios between substances and calculate the mass or volume of a substance involved in a reaction.
The Law of Conservation of Mass is a fundamental principle in chemistry, guiding the understanding and prediction of chemical reactions. It ensures that the total mass of a closed system remains constant, allowing for accurate calculations and predictions in the field of chemistry.
Law of Conservation of Mass in Modern Physics
The Law of Conservation of Mass, although fundamental to classical mechanics and chemistry, had to be modified to comply with the principles of modern physics, such as quantum mechanics and special relativity. These principles introduced the concept of mass-energy equivalence, which states that mass and energy are interchangeable.
According to Albert Einstein’s theory of relativity, mass and energy are different forms of the same underlying entity. This is expressed by Einstein’s famous equation E=mc², where E represents energy, m represents mass, and c represents the speed of light in a vacuum.
The equation shows that a small amount of mass can be converted into a large amount of energy, and vice versa. This concept is demonstrated in nuclear reactions, where a small amount of mass is converted into a large amount of energy, as observed in atomic bombs and nuclear power plants.
The Law of Conservation of Mass is still valid in everyday chemical reactions, where the conversion of mass into energy is negligible compared to the total mass involved. However, in highly energetic systems, such as nuclear reactions or particle-antiparticle annihilation, the conversion of mass into energy cannot be ignored.
In these systems, the conservation of energy, including both mass and other forms of energy, is upheld. The total energy of the system, which includes the energy associated with mass as well as other forms of energy like kinetic energy and potential energy, remains constant.
The Law of Conservation of Mass in modern physics is thus extended to the Law of Conservation of Mass-Energy, which encompasses the interconversion of mass and energy. This principle is of significant importance in understanding the behavior of matter and energy in highly energetic systems.
In conclusion, the Law of Conservation of Mass had to be modified in modern physics to incorporate the principles of mass-energy equivalence. While the Law of Conservation of Mass is still applicable in everyday chemical reactions, the interconversion of mass and energy must be considered in highly energetic systems.

Solved Examples on the Law of Conservation of Mass
The Law of Conservation of Mass is a powerful tool for solving problems in chemistry and physics. By applying the principle that mass is conserved in a closed system, we can determine the quantities of reactants and products in a chemical reaction.
Let’s solve some examples to illustrate how the Law of Conservation of Mass is applied:
Example 1: If 10 grams of calcium carbonate (CaCO3) produces 3.8 grams of carbon dioxide (CO2) and 6.2 grams of calcium oxide (CaO), show that these observations are in agreement with the Law of Conservation of Mass.
Solution: According to the Law of Conservation of Mass, the mass of the reactants must be equal to the mass of the products. Let’s calculate the total mass of the reactants and products in this reaction:
Mass of reactants = Mass of products 10 grams of CaCO3 = 3.8 grams of CO2 + 6.2 grams of CaO
As we can see, the total mass of the reactants (10 grams) is equal to the total mass of the products (3.8 grams + 6.2 grams = 10 grams). Therefore, these observations are in agreement with the Law of Conservation of Mass.
Example 2: In the reaction between hydrogen (H2) and oxygen (O2) to form water (H2O), if 4 grams of hydrogen reacts with 32 grams of oxygen, what is the mass of water produced?
Solution: To solve this problem, we need to determine the mass of water produced using the Law of Conservation of Mass. The balanced chemical equation for the reaction is:
2H2 + O2 → 2H2O
From the equation, we can see that 2 moles of water are produced for every 2 moles of hydrogen. The molar mass of hydrogen is approximately 2 grams/mol, and the molar mass of oxygen is approximately 32 grams/mol.
Therefore, the mass of water produced can be calculated as follows:
Mass of water produced = (Mass of hydrogen) × (Molar mass of water) / (Molar mass of hydrogen) = (4 grams) × (18 grams/mol) / (2 grams/mol) = 36 grams
Thus, the mass of water produced in this reaction is 36 grams.
By solving similar examples, we can verify the Law of Conservation of Mass and gain a deeper understanding of its application in chemical reactions.
How Kunduz Can Help You Learn the Law of Conservation of Mass?
Kunduz is an online learning platform that provides comprehensive and interactive resources to help you learn and understand the Law of Conservation of Mass. Whether you’re a student studying chemistry or a curious learner interested in physics, Kunduz can support your learning journey.
On Kunduz, you can find a wide range of educational materials, including video lessons, interactive quizzes, and practice problems. These resources are designed to break down complex concepts into manageable and engaging content, making it easier for you to grasp the principles of the Law of Conservation of Mass.
Kunduz offers step-by-step explanations and examples, allowing you to master the formula and application of the Law of Conservation of Mass. The platform also provides personalized recommendations and feedback, ensuring that you progress at your own pace and address any areas of difficulty.
With Kunduz, you can access high-quality educational content anytime, anywhere, making learning convenient and accessible. Whether you prefer to study independently or collaborate with peers, Kunduz provides a supportive learning community where you can connect with fellow learners and experts.
Start your journey to mastering the Law of Conservation of Mass with Kunduz. Explore the platform and discover a wealth of resources to enhance your understanding and achieve academic success.
Frequently Asked Questions on the Law of Conservation of Mass
Q1: Who gave the Law of Conservation of Mass? The Law of Conservation of Mass was formulated by Antoine Lavoisier, a French chemist, in the late 18th century. Lavoisier’s experiments demonstrated that the total mass of substances involved in a chemical reaction remains constant, even if the substances undergo physical or chemical changes.
Q2: Why is there no change in mass during chemical reactions? According to the Law of Conservation of Mass, atoms are neither created nor destroyed during a chemical reaction. The total mass of the reactants must be equal to the total mass of the products. In a closed system, the atoms are rearranged to form new compounds, but the total number of atoms and their mass remain constant.
Q3: How do you verify the Law of Conservation of Mass with an experiment? To verify the Law of Conservation of Mass with an experiment, you can perform a reaction in a closed system and measure the masses of the reactants and products. The mass of the reactants should be equal to the mass of the products, demonstrating that mass is conserved during the reaction. This experiment can be conducted using a variety of chemical reactions, such as the combustion of a substance or the reaction between acids and bases.
