Nucleosides are fundamental building blocks of nucleotides, which are the monomers that make up nucleic acids like DNA and RNA. These molecules play a crucial role in storing and transmitting genetic information. Nucleosides consist of two main components: a nitrogenous base and a sugar molecule. The nitrogenous base can be either a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil). The sugar molecule can be either ribose (in RNA) or deoxyribose (in DNA). Nucleosides are formed when a base is covalently bonded to the sugar molecule.
What is Nucleoside?
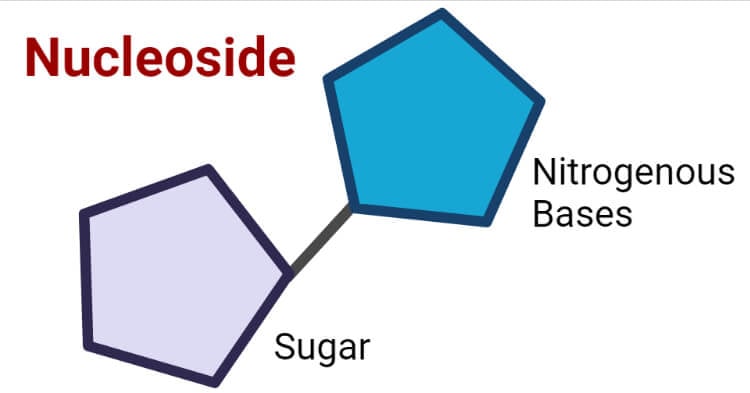
A nucleoside is a molecule composed of a nitrogenous base covalently bonded to a sugar molecule. It can be considered as a nucleotide without a phosphate group. Nucleosides are essential components of DNA and RNA, playing a vital role in the storage and transmission of genetic information.
In DNA, the nucleosides contain a 2′-deoxy-D-ribose sugar, while in RNA, the nucleosides contain D-ribose sugar. The key distinction lies in the second position of the pentose structure. In 2′-deoxyribose, there is an absence of an alcohol group (-OH) at the second position, leading to the name “2′-deoxy.” On the other hand, D-ribose has an -OH group present at the second position. Both types of pentoses exist in their β-furanose form, which is a close five-membered ring structure.
Nucleosides are considered glycosylamines since they consist of a nucleobase (also known as a nitrogenous base) and a five-carbon sugar (ribose or 2′-deoxyribose). The nucleobase can be a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil). In a nucleoside, the anomeric carbon of the sugar molecule is linked through a glycosidic bond to the N9 position of a purine base or the N1 position of a pyrimidine base.
Difference Between Nucleoside and Nucleotide
Nucleosides and nucleotides are closely related molecules, but they have distinct differences. The main difference lies in the presence or absence of a phosphate group. A nucleoside consists of a nitrogenous base covalently bonded to a sugar molecule, while a nucleotide consists of a nucleoside with one or more phosphate groups attached.
Nucleosides are the building blocks of nucleotides. When a phosphate group is covalently attached to the sugar molecule of a nucleoside, it forms a nucleotide. The addition of the phosphate group provides energy and chemical stability to nucleotides, making them essential for various cellular processes.
Another key difference between nucleosides and nucleotides is their roles in DNA and RNA. Nucleosides are the fundamental units that make up the structure of nucleic acids. They are responsible for encoding genetic information. In contrast, nucleotides participate in the synthesis and replication of DNA and RNA. They also play a crucial role in cellular signaling and energy metabolism.
Types of Nucleoside
Nucleosides can be classified based on the nitrogenous base component and the sugar component. Based on the nitrogenous base, nucleosides can be categorized into purine nucleosides and pyrimidine nucleosides. Purine nucleosides are those that contain a purine base, such as adenine or guanine, while pyrimidine nucleosides contain a pyrimidine base, such as cytosine, thymine, or uracil.
Based on the sugar component, nucleosides can be classified into ribonucleosides and deoxyribonucleosides. Ribonucleosides are nucleosides that contain ribose sugar, while deoxyribonucleosides contain deoxyribose sugar. Ribonucleosides are the building blocks of RNA, while deoxyribonucleosides are the building blocks of DNA.
In RNA, the four common ribonucleosides are adenosine, cytidine, guanosine, and uridine. Adenosine consists of the purine base adenine bonded to ribose sugar, cytidine consists of the pyrimidine base cytosine bonded to ribose sugar, guanosine consists of the purine base guanine bonded to ribose sugar, and uridine consists of the pyrimidine base uracil bonded to ribose sugar.
In DNA, the nucleosides are slightly different due to the presence of deoxyribose sugar. The four common deoxyribonucleosides are deoxyadenosine, deoxycytidine, deoxyguanosine, and deoxythymidine. Deoxyadenosine consists of the purine base adenine bonded to deoxyribose sugar, deoxycytidine consists of the pyrimidine base cytosine bonded to deoxyribose sugar, deoxyguanosine consists of the purine base guanine bonded to deoxyribose sugar, and deoxythymidine consists of the pyrimidine base thymine bonded to deoxyribose sugar.
Structure of Nucleoside
Nucleosides consist of two main components: a pentose sugar and a nitrogenous base. The pentose sugar can be either ribose (in RNA) or deoxyribose (in DNA), while the nitrogenous base can be a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil).
The structure of the pentose sugar is essential in determining whether the nucleoside is a ribonucleoside or a deoxyribonucleoside. Ribose is a five-carbon sugar with an aldehyde group at the first carbon atom. Deoxyribose is similar to ribose but lacks the hydroxyl group at the second carbon atom.
The nitrogenous base is covalently bonded to the first carbon atom (anomeric carbon) of the pentose sugar through a glycosidic bond. In a purine nucleoside, the nitrogenous base is attached to the sugar at the N9 position, while in a pyrimidine nucleoside, the nitrogenous base is attached at the N1 position.
The structure of a nucleoside is crucial for its function. The pentose sugar provides the backbone for the nucleotide, while the nitrogenous base determines the identity of the nucleoside and its role in DNA or RNA.
Components of Nucleoside
Nucleosides are composed of two main components: a nitrogenous base and a sugar molecule. The nitrogenous base can be either a purine (such as adenine or guanine) or a pyrimidine (such as cytosine, thymine, or uracil). The sugar molecule can be either ribose (in RNA) or deoxyribose (in DNA). The nitrogenous base and the sugar molecule are covalently bonded together through a glycosidic bond.
The nitrogenous base is responsible for the identity of the nucleoside. Purine bases, such as adenine and guanine, have a double-ring structure, while pyrimidine bases, such as cytosine, thymine, and uracil, have a single-ring structure. The nitrogenous bases provide the genetic information encoded in nucleic acids.
The sugar molecule, whether ribose or deoxyribose, provides the backbone for the nucleotide. Ribose is a five-carbon sugar with an aldehyde group at the first carbon atom, while deoxyribose is similar to ribose but lacks the hydroxyl group at the second carbon atom.
The covalent bond between the nitrogenous base and the sugar molecule is called a glycosidic bond. In a nucleoside, the anomeric carbon of the sugar molecule is linked to the N9 position of a purine base or the N1 position of a pyrimidine base.
The components of a nucleoside are crucial for its structure and function. The nitrogenous base determines the identity of the nucleoside, while the sugar molecule provides the backbone for the nucleotide.
Functional Role of Nucleoside
Nucleosides play important roles in various biological processes. They are the building blocks of nucleotides, which are essential components of nucleic acids (DNA and RNA). Nucleotides are involved in the storage and transmission of genetic information.
Nucleosides are also involved in energy metabolism. Adenosine triphosphate (ATP), a nucleoside triphosphate, is a major energy carrier in cells. It provides the energy required for various cellular processes, such as muscle contraction, active transport, and biosynthesis.
Nucleosides can also act as signaling molecules. Adenosine, a purine nucleoside, acts as a signaling molecule in the nervous system. It regulates various physiological processes, including sleep, pain perception, and inflammation.
In addition, nucleosides have medical importance. Nucleoside analogs are synthetic compounds that mimic natural nucleosides. They are used as antiviral and anticancer agents. Nucleoside reverse transcriptase inhibitors (NRTIs) are a class of antiviral drugs that inhibit the reverse transcriptase enzyme, preventing the replication of retroviruses such as HIV.
Furthermore, nucleosides are involved in the synthesis of nucleic acids and proteins. They provide the necessary building blocks for DNA and RNA synthesis, as well as for the synthesis of amino acids, which are the building blocks of proteins.
Overall, nucleosides play diverse and essential roles in cellular processes, including genetic information storage and transmission, energy metabolism, cellular signaling, and the synthesis of nucleic acids and proteins.
Characteristics of Nucleoside
Nucleosides possess several key characteristics that make them important molecules in biological systems:
- Building blocks of nucleotides: Nucleosides are the fundamental units from which nucleotides are formed. When a phosphate group is covalently attached to the sugar molecule of a nucleoside, it forms a nucleotide. Nucleotides are the building blocks of nucleic acids (DNA and RNA) and are essential for genetic information storage and transmission.
- Structure and function: The structure of nucleosides is crucial for their function. The nitrogenous base provides the genetic information encoded in nucleic acids, while the sugar molecule provides the backbone for the nucleotide. The combination of the nitrogenous base and the sugar molecule determines the identity of the nucleoside and its role in DNA or RNA.
- Energy carriers: Nucleoside triphosphates, such as adenosine triphosphate (ATP), are major energy carriers in cells. They provide the energy required for various cellular processes, including muscle contraction, active transport, and biosynthesis.
- Signaling molecules: Nucleosides, such as adenosine, can act as signaling molecules in the nervous system. They regulate various physiological processes, including sleep, pain perception, and inflammation.
- Medical importance: Nucleoside analogs are synthetic compounds that mimic natural nucleosides. They are used as antiviral and anticancer agents. Nucleoside reverse transcriptase inhibitors (NRTIs) are a class of antiviral drugs that inhibit the reverse transcriptase enzyme, preventing the replication of retroviruses such as HIV.
- Synthesis of nucleic acids and proteins: Nucleosides provide the necessary building blocks for the synthesis of nucleic acids (DNA and RNA) and proteins. They are essential for DNA and RNA synthesis, as well as for the synthesis of amino acids, which are the building blocks of proteins.
The characteristics of nucleosides make them vital molecules in biological systems. They are involved in various cellular processes, including genetic information storage and transmission, energy metabolism, cellular signaling, and the synthesis of nucleic acids and proteins.
Synthesis of Nucleoside
Nucleosides can be synthesized in several ways, both naturally and synthetically. In living organisms, nucleosides can be produced de novo or obtained through the diet.
De novo synthesis of nucleosides occurs primarily in the liver. It involves the stepwise assembly of nucleosides from simpler compounds. The process begins with the synthesis of the sugar molecule, which is then combined with a nitrogenous base to form a nucleoside. This process requires various enzymes and metabolic pathways.
Nucleosides can also be obtained from the diet. Nucleic acids present in foods, such as DNA and RNA, are broken down by digestive enzymes into nucleotides. Nucleotidases then cleave the phosphate groups from the nucleotides, resulting in the formation of nucleosides. These nucleosides can be absorbed by the body and used for various cellular processes.
In addition to natural synthesis, nucleosides can also be synthesized synthetically in the laboratory. Chemical synthesis is the most common method used, as it allows for the production of specific nucleosides with desired properties. Enzymatic synthesis and recombinant DNA technology are also used to synthesize nucleosides.
Synthetic nucleosides, known as nucleoside analogs, can be modified to have specific properties. They can be used as antiviral or anticancer agents, as they can interfere with the replication of viruses or the growth of cancer cells. Nucleoside analogs are designed to mimic natural nucleosides, allowing them to be incorporated into nucleic acids during replication. Once incorporated, they can disrupt the normal functioning of the nucleic acids, leading to the inhibition of viral replication or cancer cell growth.
The synthesis of nucleosides, both natural and synthetic, plays a crucial role in understanding their structure, function, and potential applications in medicine and biotechnology.
List of nucleosides and corresponding nucleobases
The following table provides a list of nucleosides and their corresponding nucleobases:
| Nucleobase | Ribonucleoside | Deoxyribonucleoside |
|---|---|---|
| Adenine | Adenosine | Deoxyadenosine |
| Guanine | Guanosine | Deoxyguanosine |
| Cytosine | Cytidine | Deoxycytidine |
| Thymine (DNA only) | – | Deoxythymidine |
| Uracil (RNA only) | Uridine | – |
These nucleosides are essential components of DNA and RNA. They play a crucial role in storing and transmitting genetic information. Adenosine and guanosine are purine nucleosides, while cytidine and uridine (or thymidine in DNA) are pyrimidine nucleosides.
The nucleosides, in turn, serve as the building blocks for nucleotides, which are the monomers that make up nucleic acids. Nucleotides consist of a nucleoside linked to one or more phosphate groups. The phosphate groups provide the necessary energy and chemical stability for the functions of nucleic acids.
Nucleoside Metabolism
Nucleosides are metabolized in various ways in living organisms. They can be produced de novo, particularly in the liver, or obtained from the diet through the ingestion and digestion of nucleic acids.
De novo synthesis of nucleosides occurs primarily in the liver. It involves the stepwise assembly of nucleosides from simpler compounds. The process begins with the synthesis of the sugar molecule, which is then combined with a nitrogenous base to form a nucleoside. This process requires various enzymes and metabolic pathways.
Nucleosides can also be obtained from the diet. Nucleic acids present in foods, such as DNA and RNA, are broken down by digestive enzymes into nucleotides. Nucleotidases then cleave the phosphate groups from the nucleotides, resulting in the formation of nucleosides. These nucleosides can be absorbed by the body and used for various cellular processes.
Once inside the cell, nucleosides can be further broken down into their subcomponents. Nucleosidases cleave the glycosidic bond between the sugar molecule and the nitrogenous base, resulting in the release of the nucleobase and the sugar molecule. These subcomponents can then be used for various biochemical processes, such as the synthesis of nucleic acids or the production of energy.
Nucleosides can also be converted back into nucleotides through the addition of phosphate groups. Nucleoside kinases are enzymes that catalyze the phosphorylation of nucleosides, resulting in the formation of nucleotides. The addition of phosphate groups provides the necessary energy and chemical stability for the functions of nucleotides.
Overall, nucleoside metabolism is a complex process that involves the synthesis, breakdown, and conversion of nucleosides. It plays a crucial role in maintaining the balance of nucleotide pools and providing the necessary building blocks for nucleic acid synthesis and other cellular processes.
Nucleoside Diagram
A nucleoside diagram provides a visual representation of the structure and components of a nucleoside. It illustrates the relationship between the nitrogenous base, the sugar molecule, and the glycosidic bond that connects them.
The diagram shows that a nucleoside consists of a nitrogenous base, which can be a purine or a pyrimidine, covalently bonded to a sugar molecule. The sugar molecule can be either ribose or deoxyribose, depending on whether the nucleoside is part of RNA or DNA. The glycosidic bond connects the nitrogenous base to the sugar molecule.
The nucleoside diagram helps to visualize the structure and components of a nucleoside, providing a clear understanding of its composition and function in nucleic acids.
Solved Examples on Nucleoside
Example 1: Identify the nucleoside that consists of the nitrogenous base thymine bonded to a ribose sugar.
Solution: The nucleoside that consists of the nitrogenous base thymine bonded to a ribose sugar is called thymidine. Thymidine is a pyrimidine nucleoside that is an essential component of DNA. It plays a crucial role in storing and transmitting genetic information.
Example 2: Describe the process of nucleoside metabolism in living organisms.
Solution: Nucleoside metabolism in living organisms involves the synthesis, breakdown, and conversion of nucleosides. The process can occur de novo or through the ingestion and digestion of nucleic acids in the diet.
De novo synthesis of nucleosides occurs primarily in the liver. It involves the stepwise assembly of nucleosides from simpler compounds. The process begins with the synthesis of the sugar molecule, which is then combined with a nitrogenous base to form a nucleoside. This process requires various enzymes and metabolic pathways.
Nucleosides can also be obtained from the diet. Nucleic acids present in foods, such as DNA and RNA, are broken down by digestive enzymes into nucleotides. Nucleotidases then cleave the phosphate groups from the nucleotides, resulting in the formation of nucleosides. These nucleosides can be absorbed by the body and used for various cellular processes.
Once inside the cell, nucleosides can be further broken down into their subcomponents. Nucleosidases cleave the glycosidic bond between the sugar molecule and the nitrogenous base, resulting in the release of the nucleobase and the sugar molecule. These subcomponents can then be used for various biochemical processes, such as the synthesis of nucleic acids or the production of energy.
Nucleosides can also be converted back into nucleotides through the addition of phosphate groups. Nucleoside kinases are enzymes that catalyze the phosphorylation of nucleosides, resulting in the formation of nucleotides. The addition of phosphate groups provides the necessary energy and chemical stability for the functions of nucleotides.
Overall, nucleoside metabolism is a complex process that involves the synthesis, breakdown, and conversion of nucleosides. It plays a crucial role in maintaining the balance of nucleotide pools and providing the necessary building blocks for nucleic acid synthesis and other cellular processes.
How Kunduz Can Help You Learn Nucleosides?
Kunduz is an online learning platform designed to help students master challenging subjects like nucleosides. With Kunduz, you can access comprehensive lessons, practice quizzes, and interactive learning materials that cover all aspects of nucleosides. Our expert instructors will guide you through the complex concepts and provide step-by-step explanations to ensure your understanding.
Kunduz offers a range of study resources to suit your learning style. Whether you prefer video lectures, written explanations, or interactive quizzes, you’ll find the materials you need to succeed. Our platform also allows you to track your progress, review difficult topics, and connect with a community of learners to exchange ideas and get support.
With Kunduz, learning nucleosides becomes engaging, efficient, and effective. Our user-friendly interface, expert instruction, and comprehensive resources make studying enjoyable and productive. Whether you’re a beginner or an advanced learner, Kunduz has the tools you need to excel in your studies.
Start your journey to mastering nucleosides with Kunduz today and unlock your full academic potential.
