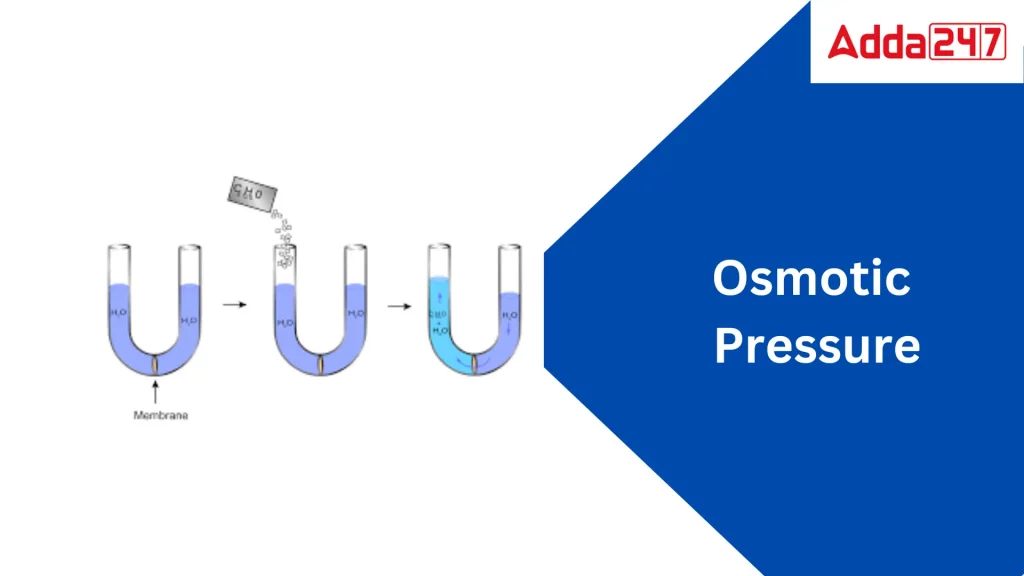
Osmotic pressure is a fundamental concept in the field of science, particularly in the study of solutions and their interactions with semi-permeable membranes. It refers to the minimum pressure required to prevent the flow of solvent molecules into a solution through a semi-permeable membrane. This pressure arises due to the natural tendency of solvents, such as water, to move from an area of lower solute concentration to an area of higher solute concentration.
Understanding osmotic pressure is crucial for various scientific disciplines, including biology, chemistry, and engineering. It plays a significant role in biological processes, such as cellular homeostasis and nutrient absorption, as well as in industrial applications like water purification and pharmaceutical development.
In this comprehensive guide, we will explore the concept of osmotic pressure in detail. We will discuss the definition, formula, and equation for osmotic pressure, as well as its significance and examples in various contexts. So, let’s dive in and unravel the mysteries of osmotic pressure!
What is Osmosis?
Before delving into osmotic pressure, it is essential to understand the process of osmosis. Osmosis is the movement of solvent molecules through a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration. The semi-permeable membrane allows the passage of solvent molecules while restricting the movement of solute particles.
The driving force behind osmosis is the natural tendency of molecules to distribute randomly, aiming for a uniform concentration throughout a given space. This phenomenon is governed by the principles of diffusion. When two solutions of varying solute concentrations are separated by a semi-permeable membrane, the solvent molecules will naturally move from the dilute solution to the concentrated one until an equilibrium is achieved.
Osmosis plays a vital role in various biological processes, including cellular hydration, nutrient absorption, and waste removal. It also has implications in other fields, such as water treatment and desalination processes. By understanding osmosis, we can grasp the underlying mechanisms that drive osmotic pressure.

What is Osmotic Pressure?
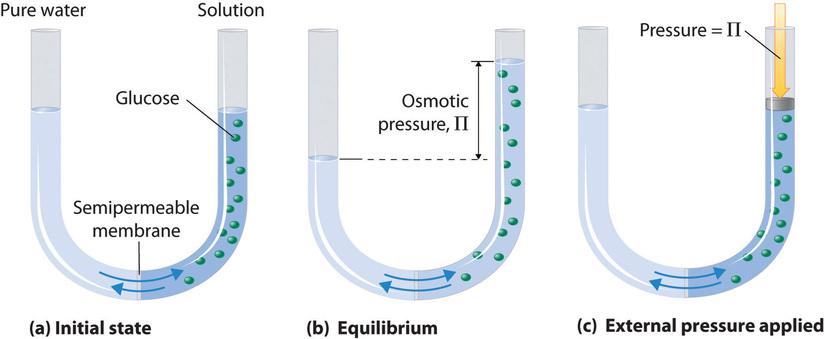
Osmotic pressure can be defined as the minimum pressure that must be applied to a solution to prevent the flow of solvent molecules into it through a semi-permeable membrane. It is a colligative property, meaning that it depends on the concentration of solute particles in the solution, rather than the specific nature of the solute.
The osmotic pressure of a solution can be calculated using the following formula:
π = iCRT
Where:
- π represents osmotic pressure
- i is the van’t Hoff factor, which represents the number of particles a solute dissociates into when dissolved in a solvent
- C is the molar concentration of the solute
- R is the universal gas constant
- T is the temperature, measured in Kelvin
This equation, proposed by the Dutch chemist Jacobus van’t Hoff, provides a mathematical relationship between osmotic pressure and other variables. It allows scientists to predict and understand osmotic behavior in various systems. It is important to note that this equation is most accurate for solutions that exhibit ideal behavior.
How to Calculate Osmotic Pressure?
Calculating osmotic pressure involves several steps. Let’s break it down into a step-by-step process:
Step 1: Determine the van’t Hoff factor (i) The van’t Hoff factor represents the number of particles a solute dissociates into when dissolved in a solvent. It is typically provided in the problem statement or can be determined from the chemical formula of the solute.
Step 2: Calculate the molar concentration (C) The molar concentration of the solute can be calculated by dividing the number of moles of solute by the volume of the solution. Make sure to convert the volume to liters if necessary.
Step 3: Convert the temperature to Kelvin (T) The osmotic pressure equation requires the temperature to be in Kelvin. To convert from Celsius to Kelvin, simply add 273 to the Celsius temperature.
Step 4: Plug the values into the osmotic pressure equation Using the values of the van’t Hoff factor (i), molar concentration (C), universal gas constant (R), and temperature (T), plug them into the osmotic pressure equation: π = iCRT
Step 5: Calculate the osmotic pressure Perform the necessary calculations to find the osmotic pressure of the solution. Make sure to include the appropriate units.
By following these steps, you can calculate the osmotic pressure of a solution accurately.
Osmotic Pressure Equation
The osmotic pressure equation provides a comprehensive framework for understanding and predicting osmotic phenomena in solutions. It incorporates variables such as the van’t Hoff factor, molar concentration, universal gas constant, and temperature, giving us a holistic view of the factors influencing osmotic pressure.
The osmotic pressure equation is represented by the formula:
π = iCRT
Where:
- π represents osmotic pressure
- i is the van’t Hoff factor
- C is the molar concentration of the solute
- R is the universal gas constant
- T is the temperature, measured in Kelvin
This equation allows scientists and engineers to calculate the osmotic pressure of a solution based on its solute concentration, temperature, and other factors. It serves as a valuable tool in various fields, from biology to industrial processes.
Derivation of the van’t Hoff formula
The osmotic pressure equation, also known as the van’t Hoff formula, was derived by the Dutch chemist Jacobus van’t Hoff in the late 19th century. Van’t Hoff’s work laid the foundation for our understanding of osmotic pressure and its relationship to solute concentration.
Van’t Hoff’s derivation of the osmotic pressure equation is based on the principles of thermodynamics and the ideal gas law. By considering the behavior of ideal gases and applying the laws of thermodynamics, he was able to develop a mathematical expression for osmotic pressure.
The derivation begins with the ideal gas law equation:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R is the gas constant
- T represents temperature
Van’t Hoff made several assumptions to adapt this equation to osmotic pressure. He assumed that the solute particles in a solution behave similarly to ideal gas particles and that the osmotic pressure is directly proportional to the concentration of solute particles.
By rearranging the ideal gas law equation and incorporating the concept of concentration, he arrived at the osmotic pressure equation:
π = iCRT
Where:
- π represents osmotic pressure
- i is the van’t Hoff factor
- C is the molar concentration of the solute
- R is the universal gas constant
- T is the temperature, measured in Kelvin
This equation provides a mathematical relationship between osmotic pressure and the concentration of solute particles in a solution. It allows scientists and engineers to calculate and understand osmotic pressure in various systems.
Osmotic Solutions
Osmotic pressure is a fundamental property of solutions that depends on the concentration of solute particles. Based on the concentration of solute, solutions can be classified into three categories: isotonic, hypertonic, and hypotonic solutions.
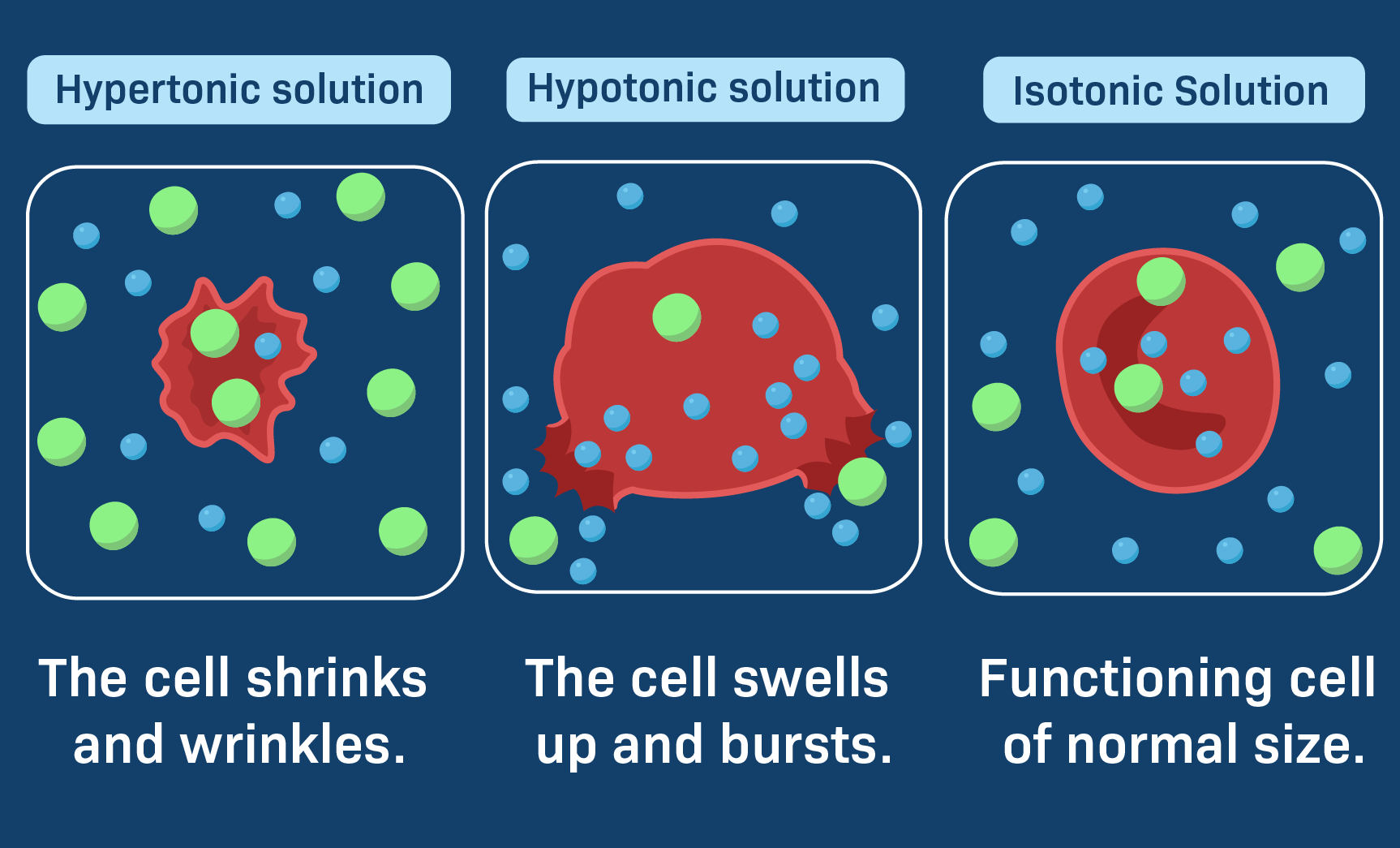
Isotonic Solution
An isotonic solution is a solution that has the same concentration of solute particles as the reference solution. In an isotonic solution, the osmotic pressure is equal to that of the reference solution. This means that there is no net movement of solvent molecules across a semipermeable membrane.
Isotonic solutions are commonly used in medical applications, such as intravenous fluids, to maintain the osmotic balance of cells and prevent cell shrinkage or swelling.
Hypertonic Solution
A hypertonic solution is a solution that has a higher concentration of solute particles compared to the reference solution. In a hypertonic solution, the osmotic pressure is greater than that of the reference solution. This creates a net movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration.
Hypertonic solutions have various applications in areas such as medicine, food preservation, and water treatment. In medicine, hypertonic saline solutions are used to reduce brain swelling and manage certain types of fluid imbalances. In food preservation, hypertonic solutions can be used to dehydrate and preserve foods by drawing out water from microorganisms. In water treatment, hypertonic solutions are employed in processes like reverse osmosis to remove impurities from water.
Hypotonic Solution
A hypotonic solution is a solution that has a lower concentration of solute particles compared to the reference solution. In a hypotonic solution, the osmotic pressure is lower than that of the reference solution. This results in a net movement of solvent molecules from an area of higher solute concentration to an area of lower solute concentration.
Hypotonic solutions find applications in various fields, including medicine and cell biology. In medicine, hypotonic solutions are used to hydrate cells and replenish fluids in cases of dehydration. In cell biology, hypotonic solutions are used to study the effects of osmotic pressure on cells and their behavior.
Understanding the different types of osmotic solutions is crucial in various scientific and practical applications. It allows us to manipulate osmotic pressure and control the movement of solvent molecules in different systems.
Types of Osmosis
Osmosis, the process of solvent molecules moving through a semipermeable membrane, can occur in various ways depending on the specific conditions. Let’s explore two important types of osmosis: endosmosis and exosmosis.
Endosmosis
Endosmosis refers to the movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration through a semipermeable membrane. In this process, solvent molecules are absorbed into the solution, resulting in an increase in the volume and pressure of the solution.
Endosmosis plays a significant role in biological systems, such as the absorption of water by plant roots and the rehydration of cells. It is also utilized in various industrial processes, such as pharmaceutical drug delivery systems and water treatment technologies.
Exosmosis
Exosmosis, also known as exosmosis or exosmose, is the opposite of endosmosis. It refers to the movement of solvent molecules from an area of higher solute concentration to an area of lower solute concentration through a semipermeable membrane. In this process, solvent molecules are released from the solution, resulting in a decrease in the volume and pressure of the solution.
Exosmosis is commonly observed in various biological processes, such as the release of water from plant leaves through stomata and the removal of excess water from animal cells. It is also utilized in industrial applications, including the dehydration of food products and the concentration of solutions.
Understanding the different types of osmosis is crucial in comprehending the mechanisms and implications of osmotic pressure in various systems.
The Principle Behind Osmotic Pressure
The principle behind osmotic pressure lies in the natural tendency of solvent molecules, especially water, to move from an area of lower solute concentration to an area of higher solute concentration. This movement occurs through a semipermeable membrane, which selectively allows the passage of solvent molecules but restricts the passage of solute particles.
The driving force behind osmosis and osmotic pressure is the desire to achieve equilibrium or equalize the concentration of solute particles on both sides of the semipermeable membrane. Solvent molecules move across the membrane until the solute concentration reaches equilibrium.
The principle of osmotic pressure is closely related to the principles of diffusion and concentration gradients. Diffusion is the process by which molecules move from an area of higher concentration to an area of lower concentration. In the case of osmosis, the movement of solvent molecules is driven by the concentration gradient of solute particles.
The presence of a semipermeable membrane is essential for the establishment of osmotic pressure. This membrane allows water molecules to pass through while preventing the passage of solute particles. As a result, the solvent molecules exert pressure on the solution side of the membrane, which is known as osmotic pressure.
Understanding the principle behind osmotic pressure helps us comprehend its significance and applications in various scientific and practical contexts. It forms the basis for designing processes such as water purification, drug delivery systems, and cellular transport mechanisms.
Types of Osmotic Pressure
Osmotic pressure can be classified into various types based on specific characteristics and conditions. Let’s explore some of the different types of osmotic pressure:
Isosmotic Pressure
Isosmotic pressure refers to the osmotic pressure of a solution that has the same solute concentration as another solution. In an isosmotic solution, the osmotic pressure is equal to the reference solution. This means that there is no net movement of solvent molecules across a semipermeable membrane.
Isosmotic pressure is important in maintaining osmotic balance in biological systems. It ensures that cells and tissues remain hydrated and function properly. It also plays a role in maintaining fluid balance in the body and regulating various physiological processes.
Hypoosmotic Pressure
Hypoosmotic pressure, also known as hyposmotic pressure, refers to the osmotic pressure of a solution that has a lower solute concentration than another solution. In a hypoosmotic solution, the osmotic pressure is lower than the reference solution. This results in a net movement of solvent molecules from an area of higher solute concentration to an area of lower solute concentration.
Hypoosmotic pressure has implications in various biological and industrial processes. In biology, it can affect cellular hydration, nutrient absorption, and waste removal. In industrial applications, hypoosmotic solutions may be utilized for certain chemical reactions or as reagents in laboratory procedures.
Hyperosmotic Pressure
Hyperosmotic pressure, also known as hyperosmolarity, refers to the osmotic pressure of a solution that has a higher solute concentration than another solution. In a hyperosmotic solution, the osmotic pressure is higher than the reference solution. This creates a net movement of solvent molecules from an area of lower solute concentration to an area of higher solute concentration.
Hyperosmotic pressure is significant in various biological processes, such as cellular dehydration and the regulation of fluid balance. It also has industrial applications, such as in the preservation of food products or the concentration of solutions.
Understanding the different types of osmotic pressure allows us to comprehend the various factors and conditions that influence osmotic behavior. It provides insights into the mechanisms and implications of osmosis in different systems.
Importance of Osmotic Pressure
Osmotic pressure plays a crucial role in various biological, chemical, and industrial processes. Let’s explore the significance of osmotic pressure in different contexts:
Cellular Health and Function
Osmotic pressure is essential for maintaining cellular health and function. Cells require a delicate balance of solute concentrations to ensure proper hydration and nutrient absorption. Any disruption in this balance can lead to cellular dehydration or swelling, which can adversely affect cell function and potentially cause cell death.
Osmotic pressure helps regulate the movement of water in and out of cells, ensuring that cells maintain their shape, size, and structural integrity. It also plays a role in the transport of ions and molecules across cell membranes, facilitating various physiological processes.
Plant Turgidity
In plants, osmotic pressure is responsible for maintaining turgor pressure, which gives plant cells their rigidity and shape. When a plant cell absorbs water, the osmotic pressure increases, exerting pressure on the cell wall and creating turgidity. This turgidity allows plants to stand upright and maintain their structural integrity.
Osmotic pressure also plays a role in the transport of water and nutrients in plants. Through osmosis, water moves from an area of lower solute concentration, such as the soil, to an area of higher solute concentration, such as the roots and leaves. This process helps plants absorb water and nutrients, supporting their growth and survival.
Kidney Function
Osmotic pressure is involved in the regulation of water and solute balance in the body, which is essential for proper kidney function. The kidneys play a crucial role in filtering waste products and excess water from the blood. Osmotic pressure helps regulate the movement of water and solutes across the walls of the kidney’s filtering units, known as nephrons.
In the kidneys, osmotic pressure assists in the reabsorption of water and essential solutes back into the bloodstream while allowing the excretion of waste products in the form of urine. This process helps maintain the body’s fluid balance and prevents excessive water loss or dehydration.
Blood Plasma Regulation
Osmotic pressure is a key factor in regulating the osmolarity of blood plasma. Blood plasma contains various solutes, such as proteins, electrolytes, and nutrients. Osmotic pressure helps maintain the balance of solutes in the blood plasma by regulating the movement of water across capillary walls.
Proteins in the blood plasma, such as albumin, exert colloid osmotic pressure, also known as oncotic pressure. This pressure helps draw water back into the capillaries, preventing excessive fluid loss from the bloodstream. Osmotic pressure plays a crucial role in maintaining blood volume, blood pressure, and overall cardiovascular health.
Nerve Function
Osmotic pressure is involved in the transmission of nerve signals and the functioning of nerve cells. Water balance in nerve cells is crucial for maintaining their electrical properties and proper communication between neurons.
Osmotic pressure helps regulate the movement of water across the nerve cell membranes, ensuring that they maintain their optimal hydration levels. Any imbalance in osmotic pressure can disrupt nerve function and potentially lead to neurological disorders.
Digestion and Nutrient Absorption
Osmotic pressure plays a role in the digestion and absorption of nutrients in the gastrointestinal tract. The process of digestion involves the breakdown of food into smaller molecules, which can then be absorbed into the bloodstream.
Osmosis helps facilitate the absorption of water and nutrients from the digestive system into the bloodstream. The movement of water and solutes across the walls of the intestines is regulated by osmotic pressure, ensuring the efficient absorption of nutrients and the elimination of waste products.
Pharmaceuticals
Osmotic pressure has implications in the pharmaceutical industry, particularly in drug delivery systems. Osmotic pressure-based drug delivery systems are designed to release drugs at a controlled rate, ensuring optimal therapeutic efficacy.
These systems utilize osmotic pressure to release drugs from a semi-permeable membrane or device. By adjusting the osmotic pressure of the system, the release rate of the drug can be controlled, providing a steady and sustained release over a specified period.
Environmental Implications
Osmotic pressure has significant environmental implications, particularly in the movement of water through soil and plant roots. Understanding osmotic pressure helps in managing irrigation systems and optimizing water use in agriculture.
Osmotic pressure also plays a role in the movement of water through geological formations, such as the flow of groundwater through porous rocks. It is an important factor in hydrology and the management of water resources.
Research and Development
Osmotic pressure is a subject of ongoing research and development in various scientific disciplines. Scientists and engineers are exploring ways to harness osmotic pressure for various applications, such as energy generation, water desalination, and drug delivery systems.
Advancements in osmotic pressure research have the potential to revolutionize industries and contribute to sustainable development. By understanding the principles of osmotic pressure, researchers can develop innovative solutions to address pressing global challenges.
Industrial Applications
Osmotic pressure has numerous industrial applications across various sectors. In the food industry, osmotic pressure is utilized in processes such as food preservation and dehydration. By exposing food products to hypertonic solutions, water is drawn out of microorganisms, preventing their growth and extending the shelf life of the food.
Osmotic pressure is also employed in water treatment technologies, such as reverse osmosis. Reverse osmosis systems utilize high osmotic pressure to remove impurities and contaminants from water, making it safe for consumption.
These are just a few examples of the significance of osmotic pressure in different fields. Understanding osmotic pressure allows scientists, engineers, and industries to develop innovative solutions, optimize processes, and improve overall efficiency.
Difference Between Hydrostatic Pressure and Osmotic Pressure
Hydrostatic pressure and osmotic pressure are two fundamental concepts in fluid dynamics and play important roles in various biological and physical systems. While they may seem similar at first, there are distinct differences between these two types of pressure. Let’s explore the difference between hydrostatic pressure and osmotic pressure.
Hydrostatic Pressure:
- Hydrostatic pressure is the pressure exerted by a fluid, such as water, when it is at rest within a confined space.
- It arises primarily due to gravitational forces acting on the fluid and is influenced by factors such as fluid density, depth, and gravitational acceleration.
- Hydrostatic pressure is described by Pascal’s law, which states that the pressure in a fluid is transmitted equally in all directions.
- In biological systems, hydrostatic pressure plays a role in processes such as blood circulation, lymphatic drainage, and the maintenance of cell shape and structure.
Osmotic Pressure:
- Osmotic pressure is the minimum pressure required to prevent the flow of solvent molecules into a solution through a semipermeable membrane.
- It arises due to differences in solute concentration on either side of the membrane and is dependent on the number of solute particles, rather than the nature of the solute.
- Osmotic pressure is a colligative property, meaning that it depends on the concentration of solute particles in the solution.
- In biological systems, osmotic pressure is involved in processes such as nutrient absorption, waste removal, and the maintenance of cellular hydration.
While both hydrostatic pressure and osmotic pressure play roles in fluid dynamics, their origins, mechanisms, and implications differ. Hydrostatic pressure is primarily influenced by gravitational forces and is related to the depth and density of the fluid. In contrast, osmotic pressure is driven by solute concentration differences and is independent of gravity. In biological systems, these pressures work together to regulate fluid balance and maintain homeostasis.
| Hydrostatic Pressure | Osmotic Pressure |
|---|---|
| Arises from gravitational forces | Arises from solute concentration differences |
| Dependent on fluid depth and density | Dependent on the number of solute particles |
| Described by Pascal’s law | Described by the osmotic pressure equation |
| Plays a role in blood circulation, lymphatic drainage, and cell structure | Plays a role in nutrient absorption, waste removal, and cellular hydration |
Understanding the difference between hydrostatic pressure and osmotic pressure is important for comprehending fluid dynamics in biological and physical systems. These pressures have distinct origins and implications, and their interplay is crucial for maintaining equilibrium and functionality in various systems.
Difference Between Turgor Pressure and Osmotic Pressure
Turgor pressure and osmotic pressure are two important concepts in plant physiology, both involving the movement of water and affecting cell health and function. While turgor pressure and osmotic pressure are related, they have distinct characteristics and roles within plant cells. Let’s explore the difference between turgor pressure and osmotic pressure.
Turgor Pressure:
- Turgor pressure refers to the hydrostatic force exerted by the cell’s fluid (cytoplasm) against its cell wall.
- It is responsible for maintaining the structural integrity and rigidity of plant cells.
- Turgor pressure is the result of water entering the cell by osmosis, causing the cell to swell and press against the cell wall.
- It provides support to the plant, allowing it to maintain an upright posture and resist the force of gravity.
- Turgor pressure is crucial for various plant functions, including nutrient uptake, cell expansion, and overall plant growth.
Osmotic Pressure:
- Osmotic pressure is the minimum pressure required to prevent the flow of solvent molecules into a solution through a semipermeable membrane.
- It arises due to differences in solute concentration on either side of the membrane and is dependent on the number of solute particles.
- Osmotic pressure plays a role in the movement of water into and out of plant cells.
- It is involved in the absorption of water from the soil by plant roots and the distribution of water throughout the plant.
- Osmotic pressure helps maintain the hydration and turgidity of plant cells, ensuring their proper functioning and growth.
While turgor pressure and osmotic pressure are related phenomena, they represent different aspects of water movement within plant cells. Turgor pressure is the result of osmotic pressure, as the entry of water into the cell by osmosis increases the pressure exerted on the cell wall. Together, turgor pressure and osmotic pressure play a crucial role in maintaining the health, structure, and function of plant cells.
| Turgor Pressure | Osmotic Pressure |
|---|---|
| Refers to hydrostatic force against the cell wall | Refers to minimum pressure to prevent solvent flow |
| Maintains cell rigidity and structure | Facilitates water movement into and out of cells |
| Dependent on water entry and cell expansion | Dependent on solute concentration differences |
| Essential for plant growth and nutrient uptake | Crucial for water absorption and distribution in plants |
Understanding the difference between turgor pressure and osmotic pressure helps in comprehending the mechanisms of water movement in plants. These pressures work together to maintain cell health, ensure proper hydration, and support plant growth.
The Osmotic Pressure of Electrolyte Solutions
Osmotic pressure is an essential parameter in electrolyte solutions, playing a significant role in various natural processes and industrial applications. Electrolyte solutions, such as saline or sports drinks, contain dissolved ions that contribute to their osmotic pressure. Understanding the osmotic pressure of these solutions aids in their effective use, from medical treatments to hydration strategies.
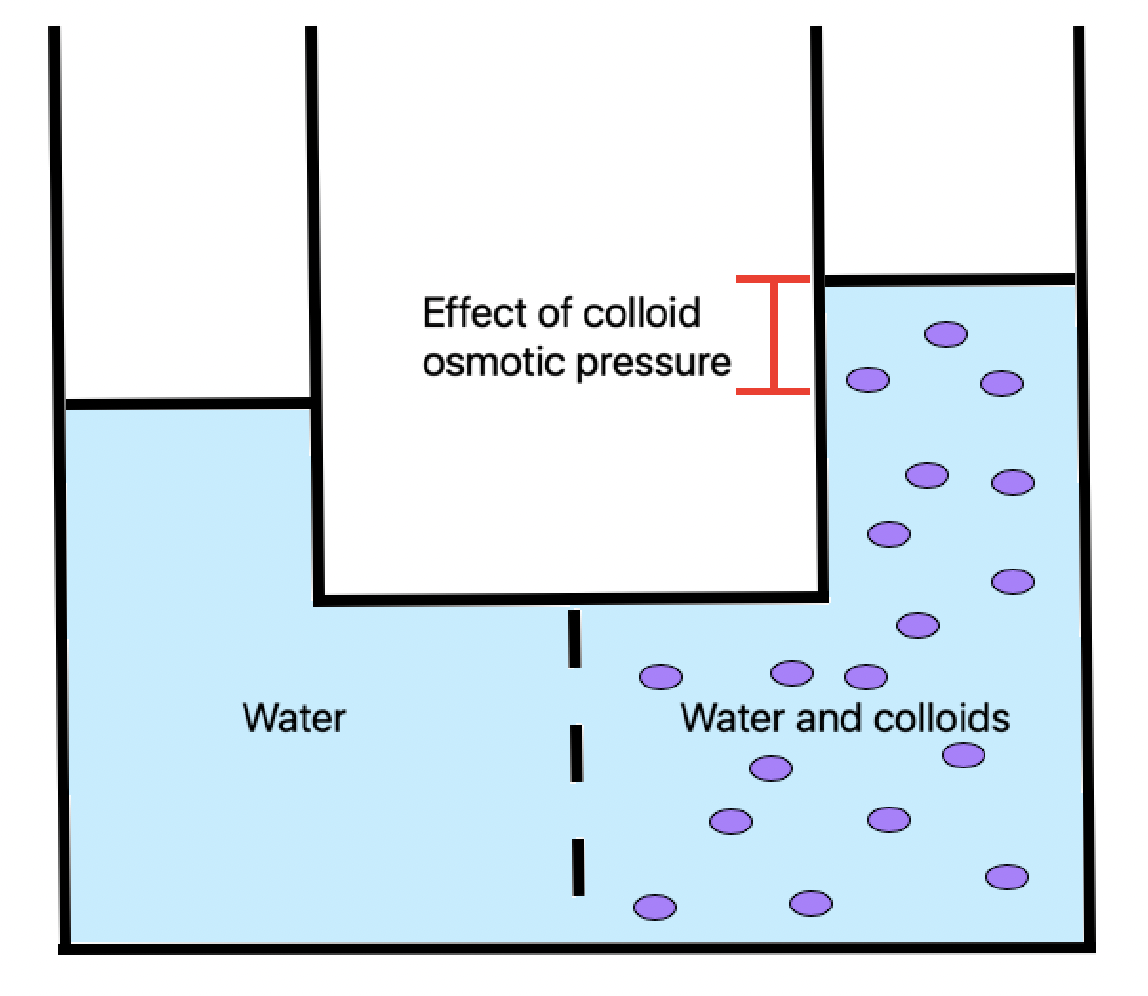
What is Colloid Osmotic Pressure?
Colloid osmotic pressure, also known as oncotic pressure, is the osmotic pressure contributed by colloids or large molecules like proteins that cannot cross a semipermeable membrane. This pressure is crucial in sustaining fluid balance within and outside cells. In human blood plasma, for instance, large proteins like albumin that cannot pass through capillary walls create an osmotic gradient that drives the movement of water from areas of lower protein concentration to areas of higher protein concentration, creating colloid osmotic pressure.
How Osmotic Pressure Works at the Molecular Level?
At a molecular level, osmotic pressure arises due to the natural propensity of molecules to distribute evenly. When a semi-permeable membrane separates a solution from its solvent, solvent molecules naturally migrate towards the solution side, seeking to equalize the concentration. This movement leads to the increased volume on the solution side and decreased volume on the solvent side, causing a difference in liquid levels across the membrane. This volume change exerts a force termed ‘osmotic pressure’, which resists further movement of solvent particles. The process ceases when the pressure induced by the increasing volume on the solution side equals the initial driving force for solvent movement.
Examples of Osmotic Pressure
Osmotic pressure is a natural process with a multitude of examples, impacting diverse areas of life and science. These include cellular functions, the preservation of food, and even the operation of certain technologies.
- In nature, osmotic pressure is a crucial factor in many biological processes. For example, plant cells utilise osmotic pressure to maintain their rigidity. The cell wall of a plant cell is semi-permeable, permitting water to move in and out. When water moves into the cell due to the higher concentration of solute particles inside, it causes the cell to swell, creating a pressure against the cell wall referred to as turgor pressure.
- In human biology, osmotic pressure is crucial in maintaining bodily homeostasis. For instance, our kidneys regulate the levels of water and salts in our bloodstream through an osmotic process. Too many salts in the blood can increase osmotic pressure, causing water to move from the body cells (where salt concentration is lower) into the bloodstream.
- Man-made systems also frequently employ osmotic pressure. For example, in food preservation, a high concentration of sugar or salt is used to create a high osmotic pressure environment. This process, known as osmotic dehydration, causes water to move out of microorganisms that might spoil the food, effectively preserving it.
- Additionally, osmotic pressure is a principle used in reverse osmosis water purification systems. These systems utilise a high pressure to overcome osmotic pressure and force water from a highly concentrated solution to a less concentrated one, effectively purifying the water from contaminants.
How Kunduz Can Help You Learn Osmotic Pressure?
Learning about osmotic pressure can be challenging due to its complex nature. However, with the right resources and guidance, you can master the concept effortlessly. By registering with Kunduz, you can access all resources and more, ensuring a well-rounded understanding of osmotic pressure and other important concepts.