Physical change is a fundamental concept in chemistry and physics that describes the alteration of a substance’s physical properties without changing its chemical composition. Understanding physical change is essential for comprehending the behavior of matter and the various transformations it can undergo. In this article, we will delve into the definition, properties, types, and examples of physical change, as well as explore the differences between physical change and chemical change.
An Introduction to Physical Change
In our everyday lives, we encounter numerous instances of physical change. From the melting of ice cubes to the boiling of water, these changes are all around us. However, it is crucial to differentiate between physical change and chemical change. While physical change affects the physical properties of a substance, chemical change involves a transformation at the molecular level, resulting in the formation of new substances.
What is Physical Change?
Physical change refers to alterations in the physical properties of a substance without any change in its chemical composition. During a physical change, the arrangement and distribution of particles within the substance may change, but the fundamental structure remains the same. In other words, the substance undergoes a transformation in its appearance, state, shape, or size, but there is no formation of new substances.
Physical Change vs. Chemical Change
To better understand physical change, it is important to contrast it with chemical change. While physical change only affects the physical properties of a substance, chemical change involves the formation of new substances with different chemical compositions. In a chemical change, bonds between atoms or molecules are broken and new bonds are formed, resulting in a complete transformation of the substance.
To summarize the differences between physical change and chemical change, let’s take a look at the following table:
| Physical Change | Chemical Change |
|---|---|
| Only affects the physical properties of a substance | Involves a change in the chemical composition of a substance |
| Reversible | Irreversible |
| No formation of new substances | Formation of new substances |
| Little to no heat or energy exchange | Heat or energy exchange may occur |
| Mass of the substance remains the same | Mass of the substance may change |
Common Physical Changes
Physical changes can manifest in various ways, altering the appearance, state, shape, or size of a substance. Let’s explore some common examples of physical changes:
Texture
Texture refers to the feel or consistency of a substance. It can change through physical processes such as sanding, polishing, or grinding. For instance, when a rough piece of wood is sanded and polished, its texture becomes smoother and more refined.
Color
Color changes are often associated with physical changes, although they can also occur in chemical changes. For example, painting a car or dyeing fabric involves a physical change in color. However, in some cases, a change in color may indicate a chemical change, such as when metal rusts or when leaves change color in the fall.

Temperature
Changes in temperature can result in physical changes. When heat is applied to a substance, it may undergo a change in state, such as melting or boiling. Conversely, when a substance loses heat, it may undergo a change in state, such as freezing or condensing.
Shape
Physical changes can also involve alterations in the shape of a substance. Folding, bending, or stretching materials like paper, metal, or rubber are examples of physical changes that result in a new shape without changing the substance’s chemical composition.
Change of State
Changes of state, such as melting, freezing, boiling, and condensing, are common examples of physical changes. These changes occur when a substance transitions between its solid, liquid, and gas states due to variations in temperature and pressure.
By understanding these common physical changes, we can further explore the characteristics and properties associated with physical change.
Characteristics of Physical Changes
Physical changes possess distinct characteristics that differentiate them from chemical changes. Let’s take a closer look at some of these characteristics:
Luster
Luster refers to the way light interacts with the surface of a substance. It is a physical property that describes the appearance of a material, ranging from shiny and reflective to dull and non-reflective. Metals often exhibit a high luster due to their ability to reflect light.
Malleability
Malleability is the property of a substance that allows it to be hammered, rolled, or pressed into various shapes without breaking. Metals, such as gold and copper, are known for their high malleability, making them ideal for shaping and forming.
Ability to be drawn into a thin wire
This property, known as ductility, refers to a substance’s capacity to be stretched into a thin wire without breaking. Metals like copper and aluminum are highly ductile and can be drawn into wires for various applications.
Density
Density is a measure of how much mass is contained within a given volume. It is a physical property that determines whether a substance will sink or float in a particular medium. Substances with higher density than the medium will sink, while those with lower density will float.
Viscosity
Viscosity is the measure of a fluid’s resistance to flow. It is a physical property that varies depending on the substance and its temperature. For example, honey has a higher viscosity than water, as it flows more slowly due to its thicker consistency.
These physical properties provide important insights into the behavior and characteristics of different substances, allowing scientists to study and understand the nature of matter.

Types of Physical Change
Physical changes can be classified into different types based on the specific transformations that occur. Let’s explore some of the most common types of physical change:
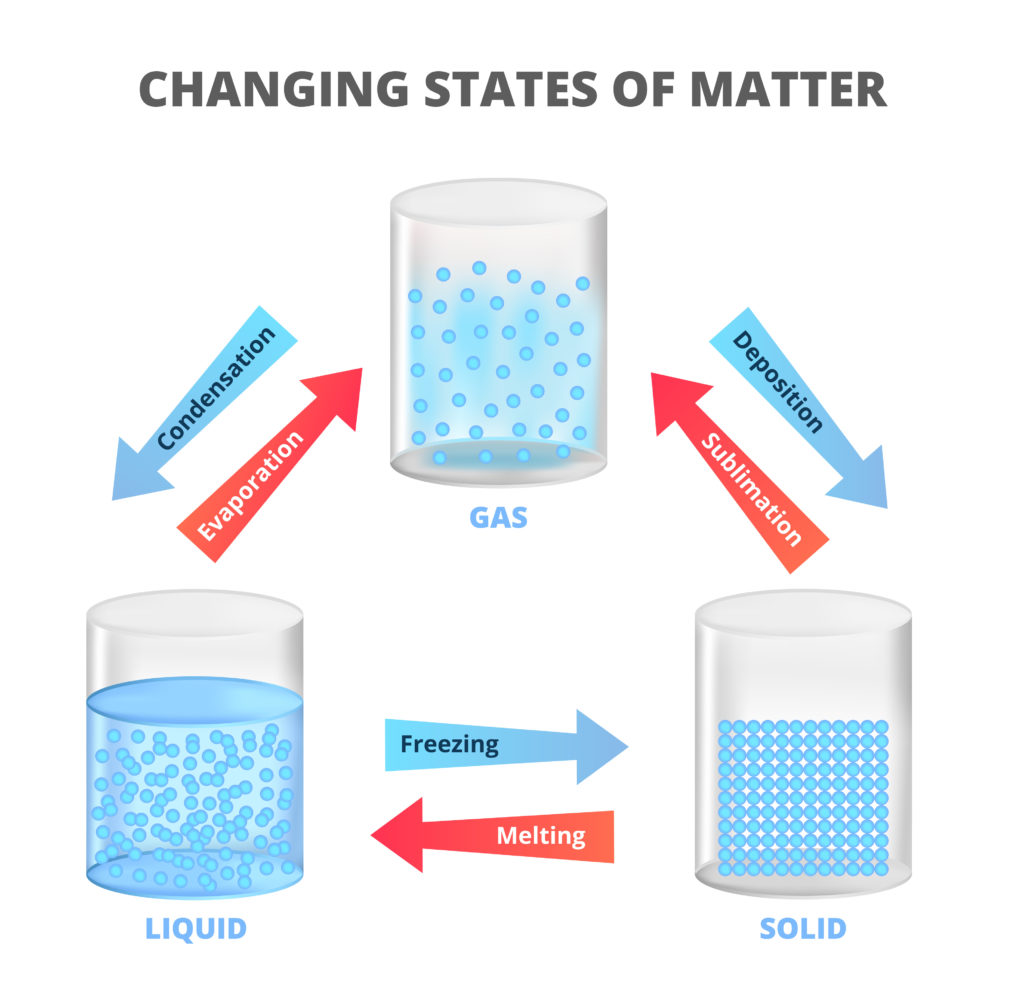
Sublimation
Sublimation is the process by which a substance transitions directly from its solid state to its gaseous state, bypassing the liquid phase. This occurs when the substance is heated and the vapor pressure exceeds the surrounding pressure. Examples of substances that undergo sublimation include dry ice (solid carbon dioxide) and mothballs (naphthalene).
Deposition
Deposition is the reverse process of sublimation, in which a substance transitions directly from its gaseous state to its solid state, bypassing the liquid phase. This occurs when the substance is cooled and the vapor pressure decreases. An example of deposition is the formation of frost on a cold surface.
Melting
Melting is the process in which a solid substance changes into a liquid state. This occurs when the substance absorbs enough heat energy to overcome the attractive forces between its particles, allowing them to move more freely. Common examples of melting include ice melting into water and wax melting when heated.
Freezing
Freezing is the reverse process of melting, in which a liquid substance transitions into a solid state. This occurs when the substance loses enough heat energy to reduce the movement of its particles, causing them to arrange in a more ordered manner. Examples of freezing include water freezing into ice and the solidification of melted chocolate.
Boiling
Boiling is the process in which a liquid substance changes into a gaseous state. This occurs when the substance is heated to its boiling point, at which the vapor pressure equals the surrounding pressure. Examples of boiling include water boiling to form steam and the evaporation of volatile liquids.
Condensation
Condensation is the reverse process of boiling, in which a gaseous substance transitions into a liquid state. This occurs when the substance loses enough heat energy to decrease the movement of its particles, causing them to come closer together. Examples of condensation include the formation of dew on grass in the morning and the fog that appears when warm air meets cold surfaces.
These types of physical change exemplify the various ways in which substances can transform between different states and phases.
Physical Change in Nature
Physical changes occur in nature on a daily basis, shaping the world around us. Natural phenomena such as weathering, erosion, and volcanic activity involve physical changes that alter the landscape and geological formations. The changing of seasons, the movement of ocean currents, and the growth of plants are all examples of physical changes occurring in nature.
What Does a Physical Change Produce?
In physical changes, no new substances are produced. The original substance undergoes a transformation in its physical properties, such as its state, shape, or size, but its chemical composition remains unchanged. This distinguishes physical changes from chemical changes, where the formation of new substances occurs through the breaking and formation of chemical bonds.
When Does a Physical Change Occur?
Physical changes can occur under various conditions, depending on the properties of the substance and the external factors involved. Changes in temperature, pressure, or the application of force can induce physical changes in matter. For example, heating ice causes it to melt, while the application of pressure can compress a gas into a liquid state.
Examples of Physical Change
To further illustrate the concept of physical change, let’s explore some examples:
Example 1: Melting Ice
When ice cubes are exposed to heat, they undergo a physical change known as melting. The ice transitions from a solid state to a liquid state, resulting in the formation of water.
Example 2: Boiling Water
When water is heated, it undergoes a physical change called boiling. The liquid water transitions into water vapor, a gaseous state.
Example 3: Dissolving Salt in Water
When salt is added to water, it dissolves and becomes distributed throughout the liquid. This is a physical change known as dissolution, where the salt particles disperse in the water without undergoing a chemical reaction.

Example 4: Stretching a Rubber Band
When a rubber band is stretched, it undergoes a physical change in shape. The rubber band elongates, but its chemical composition remains the same.
These examples highlight the different ways in which physical changes can occur and the transformations that substances can undergo without a change in chemical composition.
How Kunduz Can Help You Learn Physical Change?
At Kunduz, we are dedicated to providing comprehensive and accessible educational resources to help you understand and master the concept of physical change. We offer a range of learning materials, including informative articles, interactive videos, and step-by-step tutorials, designed to cater to various learning styles and preferences. Whether you are a student seeking additional support or a teacher looking for engaging teaching materials, Kunduz is here to assist you on your learning journey.
By exploring the principles and examples of physical change, you can develop a deeper understanding of the behavior of matter and its transformations. With Kunduz as your trusted learning companion, you can confidently navigate the world of physical science and unlock your full academic potential.
