Acid dissociation constant (pKa) is a fundamental concept in chemistry that measures the strength of an acid. It is a numerical value that represents the acidity or basicity of a molecule. Understanding pKa is crucial for predicting the behavior of acids and bases in solution.
In this article, we will explore what pKa is, how it is calculated, its relationship with pH, and its significance in various chemical processes. We will also provide a list of pKa values for common acids and answer frequently asked questions about pKa.
So, let’s dive into the world of pKa and deepen our understanding of this essential concept in chemistry.
What is pKa?
pKa is a measurement of the acidity of a compound. It is defined as the negative base-10 logarithm of the acid dissociation constant (Ka) of a solution. The pKa value indicates how tightly a proton is held by a Bronsted acid.
To put it simply, the lower the pKa value, the stronger the acid, and the more easily it donates protons. Conversely, a higher pKa value indicates a weaker acid, with a stronger hold on its protons.
The pKa value provides quantitative information about the behavior of acids and bases in solution. It is used to predict the pH of a solution when the concentration and pKa values of all acids and bases are known. Additionally, it helps calculate the equilibrium concentration of acids and bases in solution when the pH is known.
For those exploring the concept of pKa and interested in delving deeper into various chemistry topics, our ideal gas constant and benzoic acid pages serve as valuable references. They offer insights into fundamental principles such as gas behavior and acid-base properties, complementing the understanding of pKa within the broader context of chemistry.
pKa Formula
The relationship between pKa and Ka is represented by the following formula:
pKa = -log[Ka]
Here, Ka represents the acid dissociation constant. It measures the extent to which an acid dissociates in an aqueous solution. The larger the value of Ka, the stronger the acid, as it dissociates more extensively into its ions.
By taking the negative logarithm of Ka, we obtain the pKa value, which provides a convenient scale for comparing the acidity of different compounds.
pKa Equation
The pKa value can also be determined using the equilibrium concentrations of the acid and its conjugate base. Consider a weak acid, HA, which ionizes in an aqueous solution as follows:
HA (acid) + H2O ⇌ H3O+ (aq) + A– (aq) (conjugate base)
The dissociation constant of the acid, Ka, is defined as:
Ka = [H3O+] [A–] / [HA]
Taking the negative logarithm of Ka, we obtain the pKa value:
pKa = -log Ka = -log {[H3O+] [A–] / [HA]}
For polyprotic acids that contain more than one proton, the dissociation occurs stepwise, resulting in multiple pKa values. Each proton dissociation is denoted by Ka1, Ka2, Ka3, and so on.
How To Calculate pKa?
To calculate pKa, you need to know the acid dissociation constant (Ka) and the equilibrium concentrations of the acid and its conjugate base. The formula to calculate pKa is:
pKa = -log[Ka]
If you have the Ka value, you can directly calculate pKa by taking the negative logarithm of Ka.
However, if you have the equilibrium concentrations of the acid and its conjugate base, you can use the following equation:
pKa = -log {[conjugate base] / [acid]}
Simply divide the concentration of the conjugate base by the concentration of the acid and take the negative logarithm to obtain the pKa value.
Calculating pKa is essential for understanding the behavior of acids and bases in various chemical reactions and finding the optimal conditions for specific processes.
pKa and pH of Buffer Solution
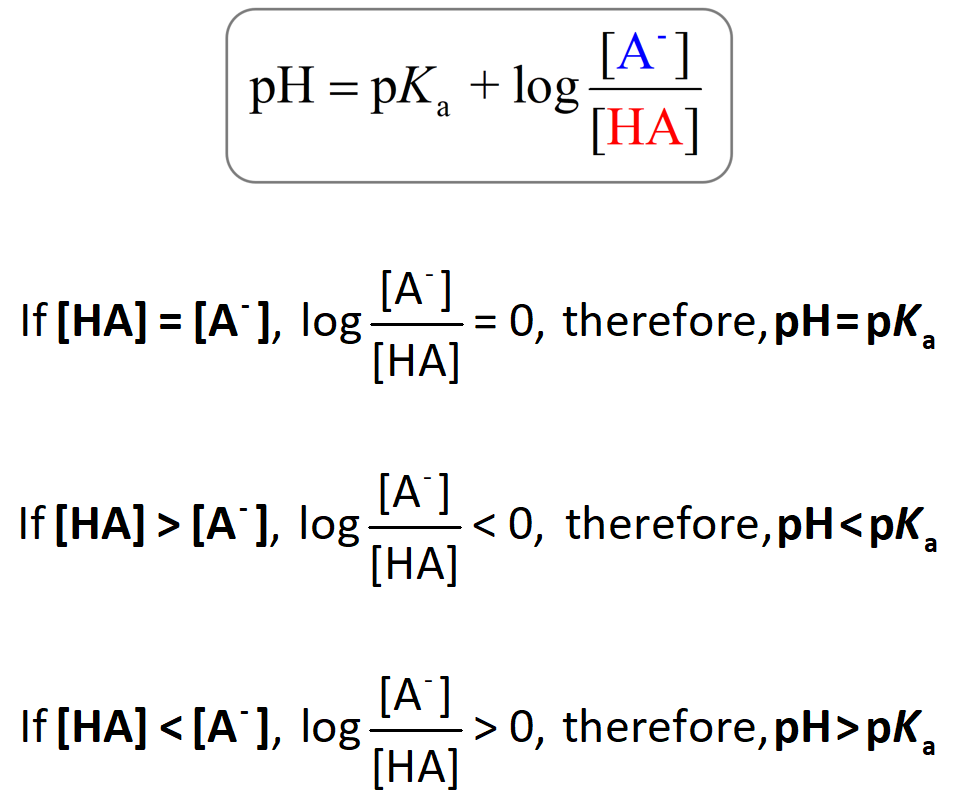
Buffer solutions play a crucial role in maintaining the pH of a solution. The Henderson-Hasselbalch equation relates the pH of a buffer solution to its pKa and the ratio of the concentration of the conjugate base to the weak acid:
pH = pKa + log {[conjugate base] / [weak acid]}
The Henderson-Hasselbalch equation allows us to predict the pH of a buffer solution based on the known pKa value and the concentrations of the conjugate base and weak acid. It also helps determine the optimal pH range for a buffer solution by adjusting the ratio of the conjugate base to the weak acid.
For example, if the ratio of the concentration of the conjugate base to the weak acid is 10, the pH is equal to pKa + 1. Conversely, if the ratio is 1/10, the pH is equal to pKa – 1. Buffer solutions are often prepared using weak acids or bases with pKa values close to the desired pH range.
Relation between pKa and pKb
In addition to pKa, there is another parameter called pKb, which represents the negative logarithm of the base dissociation constant (Kb). The relationship between pKa and pKb can be expressed as follows:
pKa + pKb = pKw
Here, pKw represents the negative logarithm of the water dissociation constant (Kw), which is equal to 14 at room temperature.
The relationship between pKa and pKb helps us understand the acidity and basicity of compounds and their ability to donate or accept protons. A lower pKa value corresponds to a stronger acid, while a lower pKb value corresponds to a stronger base.
Relationship between pKa and pH
The relationship between pKa and pH is crucial for understanding the behavior of acids and bases. pH is a measure of the concentration of hydrogen ions (H+) in a solution, while pKa represents the acidity of a compound.
The pH of a solution can be predicted by comparing the pKa values of the acids and bases present in the solution. If the pH is higher than the pKa, the solution is more basic, while if the pH is lower than the pKa, the solution is more acidic.
The Henderson-Hasselbalch equation, mentioned earlier, relates the pH of a solution to its pKa and the ratio of the conjugate base to the weak acid. This equation helps determine the pH range at which a compound can donate or accept protons.
Understanding the relationship between pKa and pH is essential for various areas of chemistry, biology, medicine, and geology. It enables accurate prediction and calculation of the behavior of acids and bases in solution.
List of pKa value of acids
pKa values vary for different acids, providing insights into their acidity or basicity. Here is a list of pKa values for some common acids:
| Acid | pKa |
|---|---|
| Acetic acid | 4.76 |
| Benzoic acid | 4.20 |
| Hydrochloric acid | -8.0 |
| Nitric acid | -1.3 |
| Sulfuric acid | -3.0 |
| Water | 15.7 |
The pKa values listed above demonstrate the range of acidity for different acids. Acetic acid, with a pKa of 4.76, is a weak acid that donates protons less easily, while hydrochloric acid, with a pKa of -8.0, is a strong acid that readily donates protons.
These pKa values are essential for understanding the behavior of acids in various chemical reactions and their impact on the overall pH of a solution.
Conversion to Ka
Conversely, if the pKa value is known, it can be converted to Ka using the following equation:
Ka = 10^(-pKa)
By taking the negative exponent of 10 to the power of the negative pKa value, we can obtain the acid dissociation constant (Ka). This allows us to compare the strength of acids based on their pKa values.
Converting pKa to Ka is useful for determining the extent to which an acid dissociates in solution and its ability to donate protons.
pKa of Some Weak & Strong Acids
The pKa values of various acids can vary widely, indicating their relative strengths. Here are some examples of weak and strong acids and their corresponding pKa values:
- Hydrocyanic acid (HCN, weak acid) – pKa = 9.21
- Acetic acid (weak acid) – pKa = 4.75
- Hydrofluoric acid (HF, weak acid) – pKa = 3.14
- Hydrochloric acid (HCl, strong acid) – pKa = -8
- Sulfuric acid (strong acid) – pKa ~ 3
These examples illustrate the range of pKa values for different acids, indicating their varying strengths. Weak acids like acetic acid and hydrofluoric acid have higher pKa values, indicating their relatively lower acidity. Strong acids like hydrochloric acid and sulfuric acid have lower pKa values, indicating their greater acidity.
Understanding the pKa values of different acids helps in predicting their behavior in chemical reactions and their impact on the overall acidity of a solution.
Ionization state vs. pKa prediction
The ionization state of a compound can often be predicted based on its pKa value. A compound with a lower pKa value is more likely to ionize in solution and donate protons. Conversely, a compound with a higher pKa value is less likely to ionize and donate protons.
The pKa value provides insight into the relative strength of an acid and its propensity to dissociate. By comparing the pKa values of different compounds, we can predict the relative acidity or basicity of molecules and their behavior in various chemical reactions.
Understanding the ionization state based on pKa prediction is essential for designing experiments and predicting the behavior of acids and bases in solution.
pKa Prediction by Atom Typing
Atom typing is a method used to predict pKa values based on the chemical structure of a compound. By analyzing the types of atoms present and their positions in the molecule, it is possible to estimate the pKa value.
Atom typing algorithms take into account various factors such as the electronegativity of atoms, their hybridization, and the presence of functional groups. These algorithms use statistical models to predict the pKa value based on these factors.
Atom typing is a powerful tool in computational chemistry and drug design, as it allows researchers to estimate the pKa values of compounds before experimental testing. This information aids in the understanding of the compound’s behavior and its potential applications in various fields.
pKa prediction by Hammett and Taft equations
The Hammett and Taft equations are mathematical models used to predict pKa values based on the substituents present in a molecule. These equations take into account the electronic and steric effects of substituents on the acidity or basicity of a compound.
The Hammett equation, developed by Louis Plack Hammett, relates the pKa value to the substituent’s electronic effect. It considers the electron-donating or electron-withdrawing nature of the substituent and its impact on the acidity of the compound.
The Taft equation, developed by Robert W. Taft, incorporates both electronic and steric effects of substituents on the acidity or basicity of a compound. It takes into account the steric hindrance caused by the substituent as well as its electronic effects.
These equations provide a quantitative approach to predict the pKa values of compounds based on their substituents, aiding in the understanding of organic chemistry and drug design.

Ionization Constants of Heteroatom Organic Acids
Heteroatom organic acids, such as carboxylic acids, amines, and phenols, have unique ionization constants due to the presence of heteroatoms like oxygen, nitrogen, and sulfur.
Carboxylic acids, characterized by the -COOH functional group, have a typical pKa range of 2-5. The ionization of carboxylic acids involves the loss of a proton from the -COOH group, resulting in the formation of a carboxylate anion.
Amines, which contain a nitrogen atom with a lone pair of electrons, exhibit varying pKa values depending on their structure. Primary amines typically have pKa values in the range of 9-11, while secondary amines have pKa values around 7-9.
Phenols, characterized by the -OH group attached to an aromatic ring, have pKa values around 10. The ionization of phenols involves the loss of a proton from the -OH group, resulting in the formation of a phenolate anion.
Understanding the ionization constants of heteroatom organic acids is crucial for studying their reactivity, understanding their behavior in solution, and designing chemical processes involving these compounds.
Frequently Asked Questions About pKa
Here are some frequently asked questions about pKa:
Is pKa equal to pH?
No, pKa is not equal to pH. pH measures the concentration of hydrogen ions (H+) in a solution, while pKa measures the strength of an acid. However, there is a relationship between pKa and pH, as the pH of a solution can be predicted based on the pKa values of the acids and bases present in the solution.
What does a high pKa mean?
A high pKa value indicates a weak acid that holds its protons tightly and does not readily donate them. It signifies a lower acidity compared to compounds with lower pKa values.
Why is pKa important?
pKa is important as it provides quantitative information about the acidity or basicity of a compound. It helps predict the behavior of acids and bases in solution, determine optimal pH ranges for buffer solutions, and design chemical processes. pKa values are widely used in various fields of chemistry, biology, medicine, and geology.
Solved Examples on pKa
Let’s solve some examples to better understand the concept of pKa.
Example 1: Calculate the pKa of acetic acid (CH3COOH) given its Ka value of 1.8 x 10^-5.
Solution: To calculate the pKa, we can use the formula pKa = -log[Ka]. Plugging in the given Ka value, we have:
pKa = -log(1.8 x 10^-5) = 4.74
Therefore, the pKa of acetic acid is 4.74.
Example 2: Find the pKa of a weak acid with a Ka value of 2.5 x 10^-9.
Solution: Using the formula pKa = -log[Ka], we can calculate the pKa as follows:
pKa = -log(2.5 x 10^-9) = 8.60
Hence, the pKa of the weak acid is 8.60.
Example 3: Determine the pH of a buffer solution with a pKa of 4.8 and a ratio of [salt]/[acid] equal to 10.
Solution: Using the Henderson-Hasselbalch equation, pH = pKa + log ([salt]/[acid]), we can substitute the given values to calculate the pH:
pH = 4.8 + log(10) = 4.8 + 1 = 5.8
Therefore, the pH of the buffer solution is 5.8.
How Kunduz Can Help You Learn pKa?
At Kunduz, we understand the importance of pKa and its role in chemistry. We offer a range of educational resources and tools to help you learn and master the concept of pKa. Our experienced teachers and comprehensive study materials provide a structured and engaging learning experience.
Whether you need assistance with understanding pKa calculations, exploring the relationship between pKa and pH, or learning about the behavior of acids and bases, Kunduz is here to guide you. Our step-by-step explanations, solved examples, and interactive learning materials ensure a thorough understanding of pKa and its applications.
Join Kunduz today and embark on a journey to unlock the fascinating world of pKa and its significance in chemistry. Let us be your trusted companion in your academic success.
