Question:
2Mg + O₂ ---> 2MgO a. Which element is the limiting reactant
Last updated: 7/7/2022
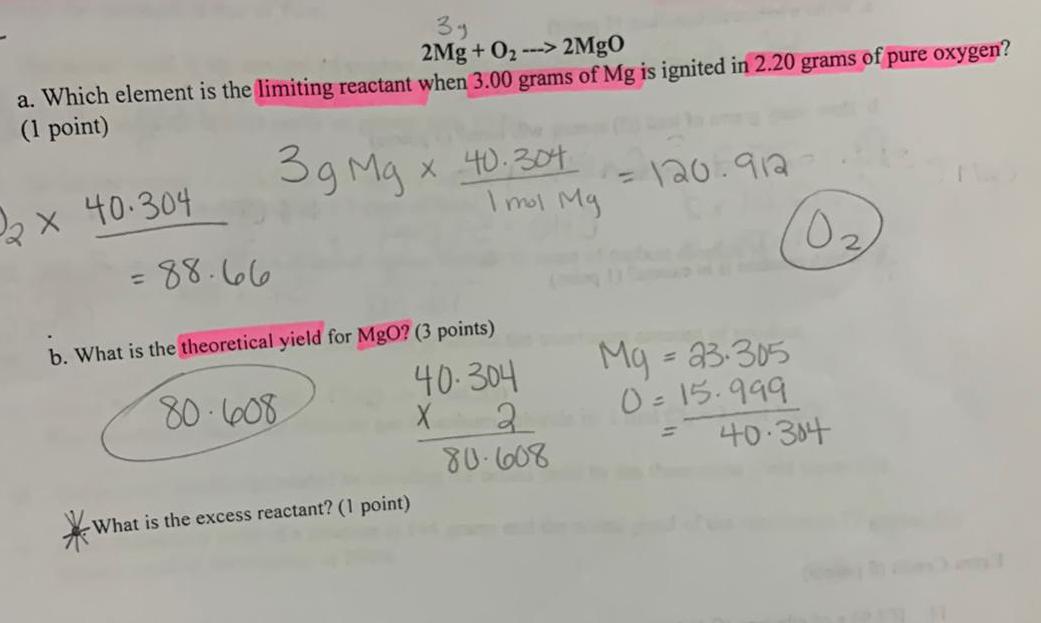
2Mg + O₂ ---> 2MgO a. Which element is the limiting reactant when 3.00 grams of Mg is ignited in 2.20 grams of pure oxygen? b. What is the theoretical yield for MgO? What is the excess reactant?