Question:
A compound is found to contain 14.88 % phosphorus and 85.13
Last updated: 7/27/2022
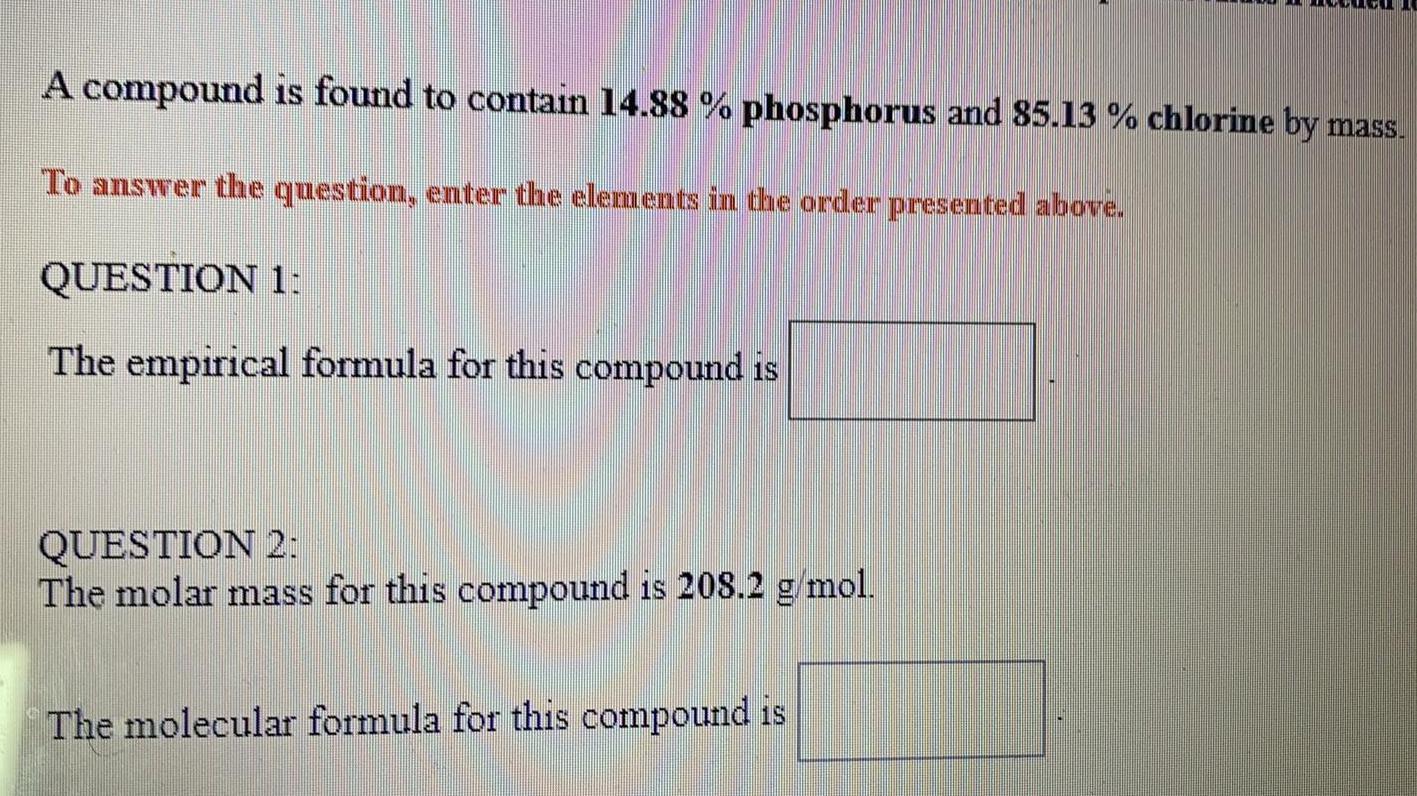
A compound is found to contain 14.88 % phosphorus and 85.13 % chlorine by mass. . QUESTION 1: The empirical formula for this compound is_____? QUESTION 2: The molar mass for this compound is 208.2 g/mol. The molecular formula for this compound is_____?