Question:
An equilibrium mixture at 300 K contains N O and NO at 0 28
Last updated: 6/30/2023
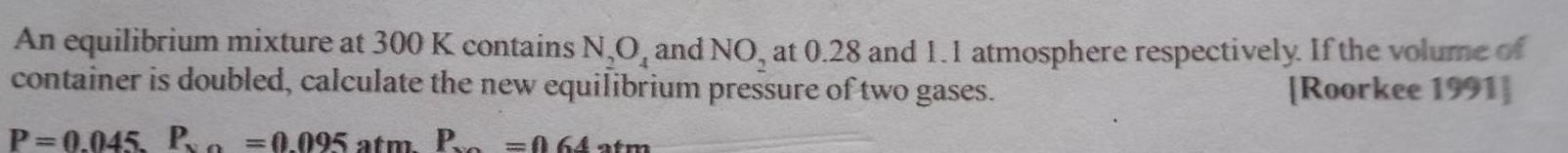
An equilibrium mixture at 300 K contains N O and NO at 0 28 and 1 1 atmosphere respectively If the volume of container is doubled calculate the new equilibrium pressure of two gases Roorkee 1991 P 0 045 P 0 095 atm Pro 064 atm