Question:
The fizz produced when an Alka-Seltzer tablet is dissolved
Last updated: 7/6/2022
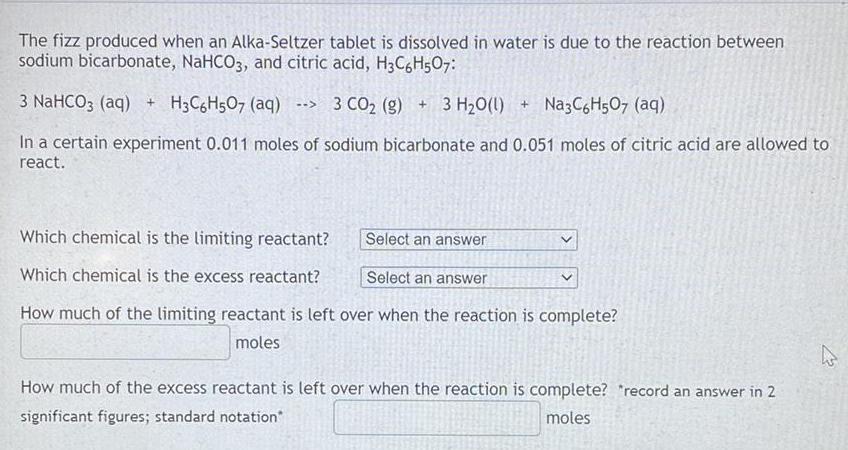
The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate, NaHCO3, and citric acid, H3C6H507: 3 NaHCO3 (aq) + H3C6H5O7 (aq) --> 3 CO₂ (g) + 3 H₂O(l) + Na3C6H507 (aq) In a certain experiment 0.011 moles of sodium bicarbonate and 0.051 moles of citric acid are allowed to react. How much of the limiting reactant is left over when the reaction is complete? Which chemical is the limiting reactant? Which chemical is the excess reactant? How much of the excess reactant is left over when the reaction is complete? "record an answer in 2 significant figures; standard notation*