The atomic radius is a fundamental concept in the realm of chemistry and physics. With its basis in the structure of an atom, the atomic radius plays a significant role in determining the properties and behavior of elements. This article delves into the essence of atomic radius, its types, the trends observed in the periodic table, and how atomic radius varies amongst elements.
An Introduction to the Atomic Radius
Atoms, the building blocks of matter, are composed of a nucleus of protons and neutrons, surrounded by a cloud of electrons. The atomic radius is a measure of the size of this electron cloud, defined as the distance from the center of the nucleus to the outermost shell where electrons reside. This distance, however, is not fixed as it depends on the energy level and distribution of the electrons. The understanding of atomic radius is crucial in studying the physical and chemical properties of elements.
Atomic Size
Atomic size and atomic radius are terms often used interchangeably. Similar to the atomic radius, atomic size relates to the distance from the nucleus to the outermost electron shell. The size of an atom is influenced by several factors, including the number of electron shells, the number of protons in the nucleus, and the overall distribution of electrons.
What is Atomic Radius?
The atomic radius is a measure that captures the size of an atom. It is typically defined as one-half the distance between the nuclei of two atoms of the same element that are bonded together. This metric allows for the comparison of sizes amongst different elements. As you move across the periodic table, the atomic radius tends to decrease from left to right across a period and increase from top to bottom down a group.
What are Atomic Radii?
The term “atomic radii” is the plural form of atomic radius. It refers to the various atomic radii measurements of different elements in the periodic table. It is important to note that atomic radii can vary within an element based on its state and the type of bond it forms with other atoms.
What is Atomic Size?
Similar to atomic radius, atomic size is a measure of the total distance from the center of an atom’s nucleus to its outermost electron shell. It is vital in understanding the properties of atoms and how they interact with each other.

Trends in the Periodic Table
The atomic radius exhibits predictable trends in the periodic table. As you move from left to right across a period, the atomic radius typically decreases. This trend is due to the increasing number of protons in the nucleus, which pulls the electrons closer to the nucleus, hence reducing the atomic radius. On the other hand, as you move down a group, the atomic radius increases. This is because of the addition of electron shells as you move down the group, leading to an increase in atomic radius.
Atomic Radius in the Periodic Table of Elements
The atomic radius can be examined graphically against the atomic number, as shown in the above chart. It provides a visual representation of how the atomic radius changes across the periodic table.
Tabular Atomic Radius (van der Waals) Data
| Atomic Number | Element | Atomic Radius (pm) |
|---|---|---|
| 1 | Hydrogen | 120 |
| 2 | Helium | 140 |
| 3 | Lithium | 182 |
| 4 | Beryllium | 153 |
| 5 | Boron | 192 |
| 6 | Carbon | 170 |
| 7 | Nitrogen | 155 |
| 8 | Oxygen | 152 |
| 9 | Fluorine | 147 |
| 10 | Neon | 154 |
The above table presents the atomic radius (van der Waals) for the first ten elements in the periodic table, measured in picometers (pm).
Types of Atomic Radius Concerning the Types of Bond
The atomic radius can be categorized based on the type of bond it forms. The three main types are covalent radius, ionic radius, and metallic radius.
Covalent Radius
The covalent radius refers to the size of an atom that is covalently bonded to another atom. It is typically measured as half the distance between the two nuclei of the bonded atoms.

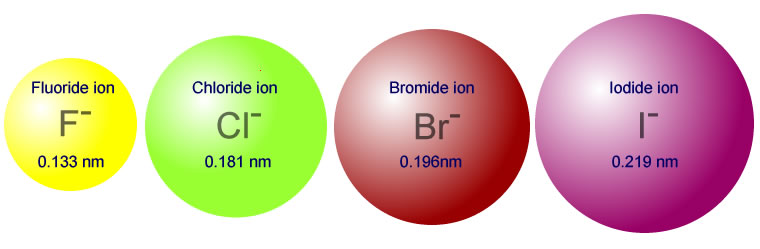
Ionic Radius
The ionic radius is a measure of the size of an ion, an atom or a group of atoms that has gained or lost electrons. It is typically measured as the distance from the center of the nucleus to the outermost shell of an ion.
Metallic Radius
The metallic radius is a measure of the size of an atom in a metallic bond. It is typically measured as half the distance between the nuclei of two adjacent atoms in a metallic lattice.
Van Der Waals Radius
The van der Waals radius is a measure of the size of an atom as determined by the distances between atoms that are not bonded but are close to each other.

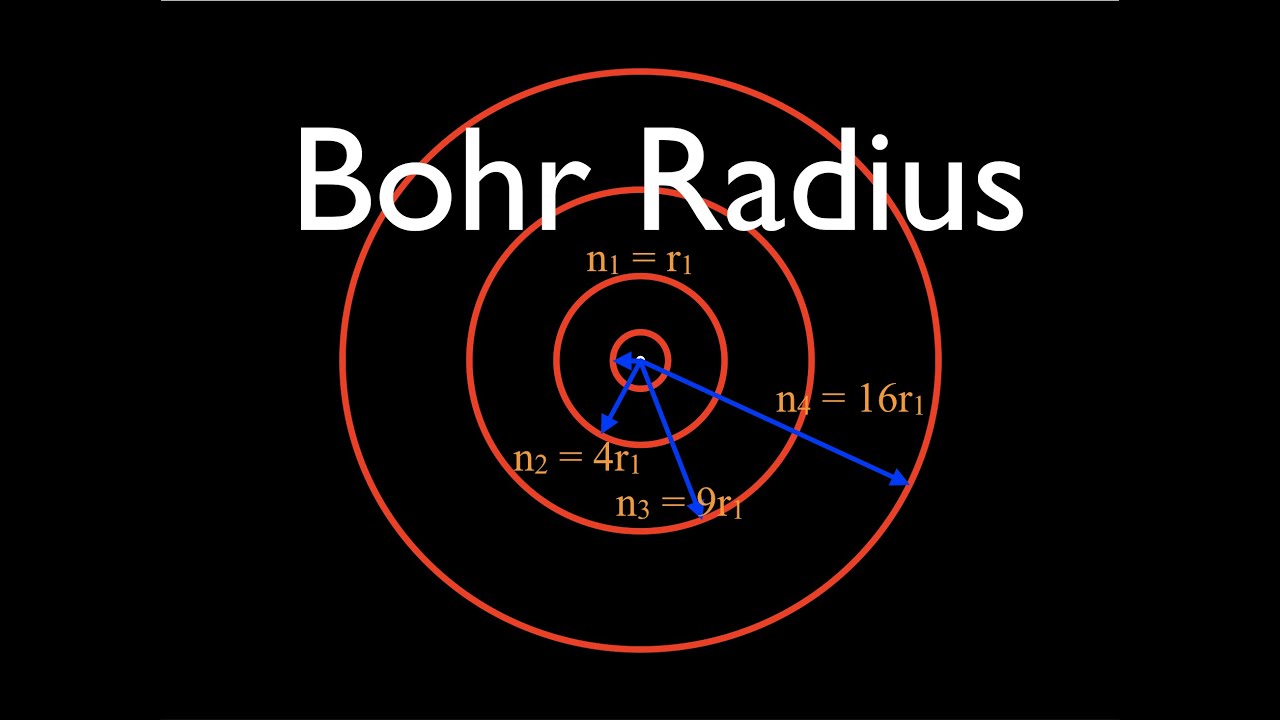
Bohr Radius
The Bohr radius is a measure of the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, who introduced the concept.
Periodic Trends in Atomic Radii
The atomic radius exhibits specific trends as you move across the periodic table. The principal factors influencing an element’s atomic radius include the nuclear charge, the number of electron shells, and the extent of shielding effect.
| Factor | Principle | Effect on Radius |
|---|---|---|
| Nuclear charge | An attractive force due to the protons in a nucleus on the electrons. | Increases with atomic number, leading to a decrease in atomic radius as you move from left to right in a period. |
| Electron shells | Determined by quantum mechanics. | Increases with the principal and azimuthal quantum numbers, leading to an increase in atomic radius as you move down a group. |
| Shielding | The repulsive force exerted by the inner electrons on the outermost shell electrons. | Decreases the effect of the nuclear charge, leading to an increase in atomic radius. |
Lanthanide Contraction
The lanthanide contraction refers to the phenomenon where the atomic radii of the elements following the lanthanides in the periodic table are smaller than expected. This effect is due to the poor shielding of the nuclear charge by the 4f electrons in the lanthanide series.
d-block contraction
Similar to the lanthanide contraction, the d-block contraction refers to the decrease in atomic size for elements in the d-block of the periodic table. This effect is due to the poor shielding of the nuclear charge by the 3d electrons in the transition metals.
Variation of Atomic Radii in the Periodic Table
There are specific trends in the atomic radii as you move across the periodic table. These trends are primarily driven by the increase in nuclear charge and the addition of electron shells.
Variation Within a Periodic Table
As you move from left to right across a period, the atomic radius typically decreases. This is due to the increase in the nuclear charge, which pulls the electrons closer to the nucleus, decreasing the atomic radius.
Variation Within a Group
As you move down a group, the atomic radius typically increases. This is due to the addition of electron shells as you move down the group, which increases the atomic radius.
Frequently Asked Questions on Atomic Radius
How To Find Atomic Radius?
The atomic radius is typically found by measuring the distance between the nuclei of two adjacent atoms and then dividing that distance by two. This can be done using X-ray crystallography or other spectroscopic methods.
Is there an increase in the atomic radius across a period?
Typically, the atomic radius decreases as you move from left to right across a period. This is due to the increase in nuclear charge, which pulls the electrons closer to the nucleus and decreases the atomic radius.
Why can’t we measure the atomic radius directly?
Direct measurement of the atomic radius is challenging due to the uncertainty in the position of the outermost electron. Furthermore, the electron cloud around the nucleus does not have a well-defined edge, making it difficult to determine the exact boundary of an atom.
What is the meaning of atomic radii?
Atomic radii refer to the various measurements of atomic radius for different elements in the periodic table. It is important to note that atomic radii can vary within an element based on its state and the type of bond it forms with other atoms.
Which element has the largest atomic radius?
Francium, located in the lower left corner of the periodic table, is the element with the largest known atomic radius.
Solved Examples on Atomic Radius
Example 1: Calculate the atomic radius of a lithium atom, given that the distance between adjacent atoms in lithium metal is 265 pm.
Solution: The atomic radius is typically half the distance between adjacent atoms. Therefore, the atomic radius of a lithium atom is 265 pm / 2 = 132.5 pm.
Example 2: If the atomic radius of a magnesium atom is 160 pm, what is the distance between adjacent magnesium atoms in magnesium metal?
Solution: The distance between adjacent atoms is typically twice the atomic radius. Therefore, the distance between adjacent magnesium atoms is 160 pm x 2 = 320 pm.
Example 3: The atomic radius of a chlorine atom is 100 pm. What is the atomic radius of a chloride ion?
Solution: When a chlorine atom gains an electron to become a chloride ion, the added electron increases electron-electron repulsion and causes the electron cloud to expand. Therefore, the atomic radius of a chloride ion is greater than 100 pm. The exact value depends on the specific environment of the ion.
How Kunduz Can Help You Learn Atomic Radius?
Understanding the concept of atomic radius can be challenging, given its abstract nature. But don’t worry, Kunduz is here to help you grasp this fundamental concept in chemistry. Kunduz provides comprehensive learning materials, engaging quizzes, and expert guidance to ensure you have a solid understanding of atomic radius and its importance in studying the properties of elements. With Kunduz, you can conquer the world of atomic radius and beyond!
For readers exploring the concept of atomic radius and interested in broader chemistry topics, our benzoic acid and Boltzmann constant pages serve as valuable references. These resources offer insights into the chemical properties of benzoic acid and the fundamental role of the Boltzmann constant, providing a well-rounded understanding that complements the exploration of dipole moments within the broader context of chemical principles.
