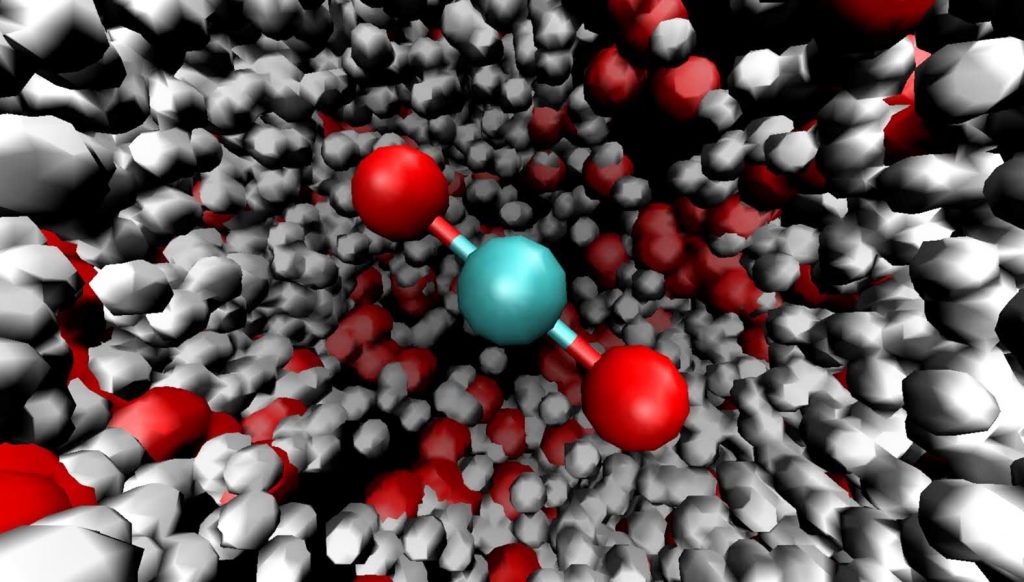
Carbonic acid, with the chemical formula H2CO3, is a carbon-containing compound that plays a crucial role in various natural and artificial processes. It is known as a weak acid and is formed when carbon dioxide (CO2) dissolves in water. In this comprehensive guide, we will explore the structure, properties, preparation methods, and uses of carbonic acid, as well as its significance in blood and oceans.
For readers exploring the concept of dipole moments and interested in broader chemistry topics, our benzoic acid and Boltzmann constant pages serve as valuable references. These resources offer insights into the chemical properties of benzoic acid and the fundamental role of the Boltzmann constant, providing a well-rounded understanding that complements the exploration of dipole moments within the broader context of chemical principles.
An Introduction to Carbonic Acid (H2CO3)
Carbonic acid is a compound that consists of hydrogen (H), carbon (C), and oxygen (O) atoms. It is an important chemical in our daily lives, with applications ranging from the production of carbonated beverages to the transportation of carbon dioxide out of our bodies. Understanding the structure and properties of carbonic acid is essential for comprehending its various uses and effects.
What is Carbonic Acid?
Carbonic acid, with the chemical formula H2CO3, is a weak acid that is formed when carbon dioxide dissolves in water. It can also be referred to as dihydrogen carbonate, acid of air, or aerial acid. Carbonic acid plays a crucial role in the natural leaching process in temperate ecosystems. It is also the only acid exhaled in the gaseous state by the human lungs, earning it the title of a respiratory acid.
Carbonic Acid Structure
The structure of carbonic acid consists of one carbon-oxygen double bond and two carbon-oxygen single bonds. The oxygen atoms in the single bonds each have one hydrogen atom attached to them. The carbonic acid molecule can also be represented as OC(OH)2, highlighting the presence of the carbon-oxygen double bond.

Carbonic Acid Lewis Structure
The Lewis structure of carbonic acid illustrates the arrangement of atoms and lone pairs of electrons in the molecule. In the Lewis structure, the carbon atom is surrounded by two oxygen atoms and two hydrogen atoms. The double bond between carbon and one oxygen atom and the single bonds between carbon and the other oxygen atom and hydrogen atoms are represented by lines.

Carbonic Acid Formula
The chemical formula of carbonic acid is H2CO3. This formula indicates that each molecule of carbonic acid consists of two hydrogen atoms (H), one carbon atom (C), and three oxygen atoms (O). The presence of the hydrogen atoms makes carbonic acid a weak acid, capable of donating protons (H+) in aqueous solutions.
Carbonic Acid Equation
The formation of carbonic acid can be represented by the following equation:
CO2 + H2O ⇌ H2CO3
In this equation, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3). The reaction is reversible, meaning that carbonic acid can also dissociate back into carbon dioxide and water. This equilibrium is important in natural processes such as the transport of carbon dioxide in the blood and the absorption of carbon dioxide by the oceans.
Properties of Carbonic Acid
Physical Properties:
- Molar Mass: The molar mass of carbonic acid is approximately 62.03 grams per mole.
- Density: In its standard state, carbonic acid has a density of about 1.67 grams per cubic centimeter.
- pH: The pH of carbonic acid is around 4.68 in a 1 millimolar (mM) solution.
- Solubility: Carbonic acid is soluble in water, and the solubility increases with higher carbon dioxide concentrations.
Chemical Properties:
- Weak Acid: Carbonic acid is classified as a weak acid because it only partially dissociates in water, releasing a small number of hydrogen ions (H+).
- Diprotic Acid: It is a diprotic acid, meaning it can donate two protons (H+) per molecule. The first dissociation occurs more readily than the second dissociation.
- Formation of Salts: Carbonic acid can form two types of salts, bicarbonates and carbonates, through reactions with bases.
Uses of Carbonic Acid
Carbonic acid has various practical applications in different industries and processes. Some of the common uses of carbonic acid are:
- Carbonated Beverages: Carbonic acid is used in the production of carbonated beverages, such as soda and sparkling water. It provides the characteristic fizz and tangy taste in these drinks.
- pH Buffer: Carbonic acid and its bicarbonate/carbonate conjugate base act as a pH buffer system in biological systems. This buffer system helps maintain a stable pH in the blood and other bodily fluids.
- pH Control: Carbonic acid is used in various industries, such as water treatment and swimming pools, to adjust and control the pH levels.
- Leavening Agent: Carbonic acid is used as a leavening agent in baking. When combined with heat, carbonic acid decomposes, releasing carbon dioxide gas, which helps dough rise and create a light texture in baked goods.
- Precipitation of Salts: Carbonic acid is involved in the precipitation of certain salts, such as ammonium persulfate, which is widely used in laboratory and industrial applications.
- Cleaning Agent: Solutions containing carbonic acid are effective in cleaning and removing deposits from surfaces, including contact lenses.
- Medicinal Uses: Carbonic acid has been used in medicinal practices, such as inducing vomiting in cases of drug overdose.

Importance of Carbonic Acid in Blood
Carbonic acid plays a vital role in the transportation of carbon dioxide out of the body through the respiratory gas exchange process. When we inhale oxygen, our cells produce carbon dioxide as a waste product. Carbonic acid is formed in the red blood cells through the reaction between carbon dioxide and water, catalyzed by the enzyme carbonic anhydrase. This reaction leads to the formation of bicarbonate ions, which are then transported in the blood plasma to the lungs, where they are converted back into carbon dioxide and exhaled.
Importance of Carbonic Acid in Oceans
The absorption of excess carbon dioxide from the atmosphere by the oceans has led to a phenomenon known as ocean acidification. Carbonic acid is formed when carbon dioxide reacts with seawater, causing a decrease in the pH of the ocean water. This change in pH can have significant impacts on marine life, including the ability of organisms such as corals and shellfish to build their shells and skeletons.
Effect of Carbonic Acid on Our Body
Carbonic acid has both direct and indirect effects on our body. The direct effect is related to its role in maintaining the acid-base balance in bodily fluids, while the indirect effect is associated with its involvement in various physiological processes, such as respiration. Imbalances in carbonic acid levels can lead to acid-base disorders, such as respiratory acidosis or alkalosis.
Preparation Of Carbonic Acid
Carbonic acid can be prepared by dissolving carbon dioxide gas in water. When carbon dioxide is bubbled through water, it reacts with water molecules to form carbonic acid. This reaction is reversible, and the concentration of carbonic acid depends on factors such as temperature and pressure.
pH of Carbonic Acid
The pH of carbonic acid is approximately 4.68 in a 1 millimolar (mM) solution. This acidic pH indicates that carbonic acid is a weak acid. The pH scale measures the acidity or alkalinity of a solution, with values below 7 indicating acidity.
The molecular mass of Carbonic Acid
The molecular mass of carbonic acid is approximately 62.03 grams per mole. The molecular mass is calculated by summing the atomic masses of all the atoms in the molecule. In the case of carbonic acid, it consists of one carbon atom with a mass of 12.01 atomic mass units (amu), two hydrogen atoms with a mass of 1.01 amu each, and three oxygen atoms with a mass of 16.00 amu each.
Benefits of Carbonic Acid
Carbonic acid offers several benefits in various applications. Some of the key benefits include:
- Carbonic acid can provide a refreshing and effervescent sensation in beverages.
- It helps maintain the acid-base balance in the body’s fluids.
- Carbonic acid is involved in vital physiological processes such as respiration and pH regulation.
- It contributes to the formation of shells and skeletons in marine organisms.
- Carbonic acid is used in cleaning solutions for contact lenses and other surfaces.
- It plays a crucial role in the transportation of carbon dioxide out of the body.
Frequently Asked Questions About Carbonic Acid
Is Carbonic Acid a Strong Acid?
No, carbonic acid is considered a weak acid. It only partially dissociates in water, releasing a small number of hydrogen ions (H+).
Is carbonic acid dangerous?
Carbonic acid is not considered toxic or dangerous to human health since it is naturally present in the human body. However, exposure to high concentrations of carbonic acid can irritate the respiratory tract and the eyes.
Where is carbonic acid found?
Carbonic acid is found in small amounts in the human body, carbonated beverages, rainwater, and natural bodies of water.
How is carbonic acid formed?
Carbonic acid is formed when carbon dioxide dissolves in water. This reaction occurs naturally in various processes, such as respiration and the absorption of carbon dioxide by the oceans.
Solved Examples on Carbonic Acid
Example 1: Calculate the pH of a 0.1 M solution of carbonic acid.
Solution: To calculate the pH of the carbonic acid solution, we need to consider the dissociation of the acid. Carbonic acid is a weak acid, so we can assume that it only partially dissociates. The dissociation equation is as follows:
H2CO3 ⇌ H+ + HCO3-
Since the concentration of carbonic acid is 0.1 M and assuming that x is the concentration of H+, the equilibrium expression can be written as:
x^2 / (0.1 – x) = Ka
Where Ka is the acid dissociation constant for carbonic acid.
By solving this equation, we can determine the concentration of H+ and then calculate the pH of the solution.
Example 2: Explain the role of carbonic acid in maintaining the acid-base balance in the blood.
Solution: Carbonic acid plays a crucial role in maintaining the acid-base balance in the blood through the bicarbonate buffer system. When carbon dioxide is produced as a waste product in our cells, it diffuses into the red blood cells. Inside the red blood cells, carbonic anhydrase enzyme catalyzes the reaction between carbon dioxide and water to form carbonic acid. This reaction is reversible, and carbonic acid quickly dissociates into bicarbonate ions and hydrogen ions.
H2CO3 ⇌ HCO3- + H+
The bicarbonate ions act as a base, while the hydrogen ions act as an acid. This bicarbonate buffer system helps regulate the pH of the blood by accepting or donating hydrogen ions as needed. If the blood becomes too acidic, the bicarbonate ions can accept hydrogen ions to restore balance. Conversely, if the blood becomes too alkaline, the bicarbonate ions can release hydrogen ions to lower the pH. This buffering action helps maintain the blood’s pH within a narrow range, ensuring optimal physiological function.
How Kunduz Can Help You Learn Carbonic Acid?
Kunduz is committed to helping students learn and understand complex concepts in chemistry, including carbonic acid. With our comprehensive study materials, interactive lessons, and expert guidance, you can gain a deep understanding of carbonic acid’s structure, properties, and uses. Our team of experienced educators is always available to answer your questions and provide personalized support. Start your learning journey with Kunduz today and unlock your full potential in chemistry.