In the world of chemistry, understanding the structure of molecules is essential for comprehending their properties and behaviors. One such molecule is methane (CH4), which is a colorless and odorless gas. Methane is the primary component of natural gas and is widely used as a fuel for heating, cooking, and electricity generation. It is also an important greenhouse gas and plays a significant role in climate change. The structure of methane, known as the Lewis structure, provides insights into the arrangement of atoms and the distribution of electrons in the molecule.
What is Carbon?
To understand the Lewis structure of methane, it is important to know the characteristics of the atoms involved. Carbon (C) is a chemical element with the atomic number 6 and is located in Group 14 of the periodic table. It is a nonmetal and is known for its versatility in forming compounds due to its ability to form covalent bonds. In the case of methane, carbon serves as the central atom, surrounded by four hydrogen atoms.
What is Hydrogen?
Hydrogen (H) is the lightest and simplest element in the periodic table, with an atomic number of 1. It is a colorless and odorless gas and is the most abundant element in the universe. Hydrogen is highly reactive and readily forms bonds with other elements, including carbon, to form various compounds. In the case of methane, hydrogen atoms bond with carbon to create a stable molecule.
Lewis Structure of Methane (CH4)
The Lewis structure is a visual representation of the arrangement of atoms and valence electrons in a molecule. It helps us understand the bonding and electron distribution in the molecule. In the case of methane, the Lewis structure shows one carbon atom bonded to four hydrogen atoms. The central carbon atom is surrounded by four lines, representing the covalent bonds with the hydrogen atoms.
The Lewis structure of methane can be written as follows:
H H
\ /
C
|
H
What are the Valence Electrons?
Valence electrons are the electrons present in the outermost shell of an atom. They are involved in bonding and determine the chemical properties of the atom. In the case of carbon, the valence electron configuration is 2s2 2p2, meaning it has four valence electrons. Hydrogen, on the other hand, has one valence electron.
The Basics of Lewis Structures
To draw the Lewis structure of methane, we follow a set of guidelines:
- Count the total number of valence electrons in the molecule. In the case of methane, we have one carbon atom with four valence electrons and four hydrogen atoms with one valence electron each, giving us a total of eight valence electrons.
- Determine the central atom. In methane, carbon is less electronegative than hydrogen and can form more bonds, making it the central atom.
- Connect each outer atom to the central atom with a single bond. In methane, each hydrogen atom forms a single bond with the carbon atom.
- Distribute the remaining valence electrons around the other atoms to satisfy the octet rule. In methane, each hydrogen atom now has a full outer shell with two electrons, and the carbon atom also has a full outer shell with eight electrons.
How to Draw Lewis Structure of Methane (CH4)?
Drawing the Lewis structure of methane involves a step-by-step process. Let’s walk through the process:
Step 1: Count the total number of valence electrons
In methane, the carbon atom has four valence electrons, and each hydrogen atom has one valence electron. Since there are four hydrogen atoms, we have a total of eight valence electrons to work with.
Step 2: Determine the central atom
Carbon is less electronegative than hydrogen and can form more bonds, so it will be the central atom in the structure.
Step 3: Connect each outer atom to the central atom with a single bond
In methane, each hydrogen atom forms a single bond with the carbon atom. This gives us four single bonds in total.
Step 4: Distribute the remaining valence electrons
Starting with the outer atoms (hydrogen), distribute the remaining valence electrons around them to satisfy the octet rule. Each bond between carbon and hydrogen will use two valence electrons. Since we have four C-H bonds in methane, all eight valence electrons are used up in forming these bonds.
Step 5: Check the stability of the Lewis structure
Every atom in methane has a full outer shell, satisfying the octet rule. The Lewis structure is stable and represents the arrangement of atoms and electrons in the molecule.
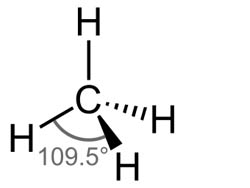
Molecular Geometry of Methane (CH4)
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. In the case of methane, the molecule has a tetrahedral geometry. Each hydrogen atom is positioned at the corner of a tetrahedron, with the carbon atom at the center. The bond angles between the carbon and hydrogen atoms are approximately 109.5 degrees.
Molecular Orbital Diagram of Methane (CH4)
A molecular orbital diagram provides insights into the mixing and overlapping of atomic orbitals to form molecular orbitals. In the case of methane, the carbon atom’s 2s orbital and three 2p orbitals (2px, 2py, and 2pz) combine to form four sp3 hybrid orbitals. These hybrid orbitals have equal energy and shape, and each can overlap with a hydrogen 1s orbital to form a sigma bond.
Hybridization in Methane (CH4)
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals. In methane, the carbon atom undergoes sp3 hybridization, which means that one 2s orbital and three 2p orbitals combine to form four sp3 hybrid orbitals. These hybrid orbitals are involved in the formation of sigma bonds with the hydrogen atoms.
Polarity and Dipole Moment of Methane (CH4)
Polarity refers to the unequal distribution of charge within a molecule. In methane, the carbon-hydrogen bonds are nonpolar because carbon and hydrogen have similar electronegativities. Additionally, methane is a symmetric molecule, meaning that the dipoles of the C-H bonds cancel each other out, resulting in a nonpolar molecule.
Methane (CH4) Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the case of methane, each hydrogen atom shares one electron with the carbon atom, and the carbon atom shares one electron with each hydrogen atom. This sharing of electrons allows each atom to achieve a stable octet.
Methane (CH4) Bond Angles
The bond angles in methane are approximately 109.5 degrees. This angle arises due to the tetrahedral geometry of the molecule. The four hydrogen atoms are positioned symmetrically around the central carbon atom, resulting in bond angles of approximately 109.5 degrees.
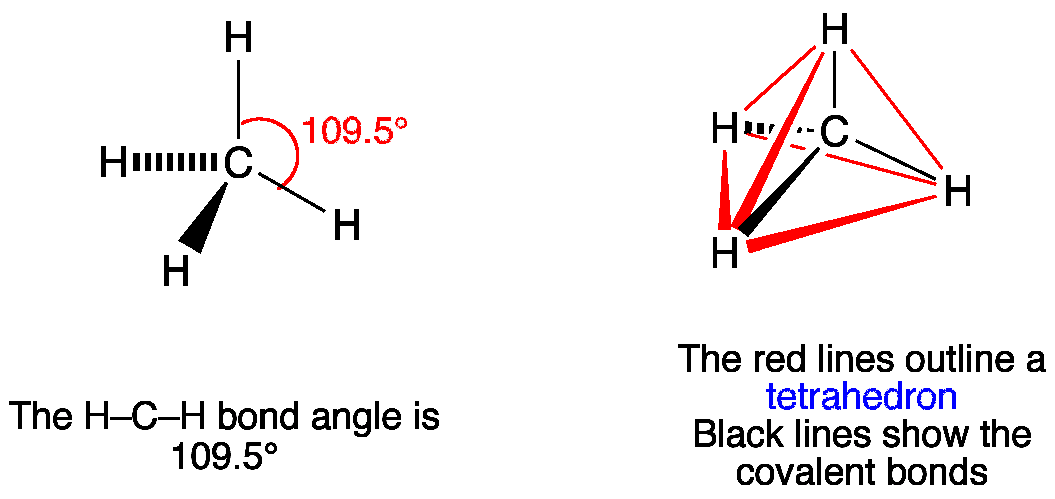
Resonance Structure of Methane (CH4)
Resonance structures are alternate Lewis structures that represent the delocalization of electrons within a molecule. In the case of methane, there are no resonance structures because the molecule does not exhibit delocalized electrons or multiple bonding.
Geometrical Structure of Methane (CH4)
The geometrical structure of methane is tetrahedral. This means that the molecule forms a three-dimensional shape resembling a pyramid with a triangular base. The carbon atom is positioned at the center of the tetrahedron, with the four hydrogen atoms at the corners.
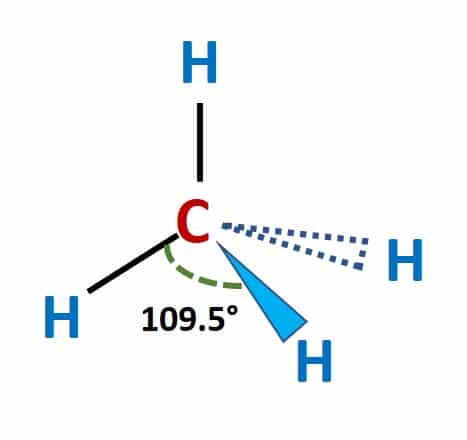
Solved Examples on Lewis Structure of Methane (CH4)
Let’s solve some examples to better understand the Lewis structure of methane:
Example 1: Draw the Lewis structure of methane (CH4).
Solution:
- Determine the total number of valence electrons: Carbon (C) = 4 valence electrons Hydrogen (H) = 1 valence electron x 4 = 4 valence electrons Total = 4 + 4 = 8 valence electrons
- Place the carbon atom in the center and connect it with four hydrogen atoms using single bonds.
- Distribute the remaining valence electrons to satisfy the octet rule. Each hydrogen atom should have a total of 2 electrons, while carbon should have a total of 8 electrons.
- Check the stability of the structure by calculating the formal charge on each atom. In methane, carbon has a formal charge of 0, and each hydrogen atom also has a formal charge of 0.
Example 2: Determine the molecular geometry of methane (CH4).
Solution: The molecular geometry of methane is tetrahedral. This means that the four hydrogen atoms are arranged symmetrically around the central carbon atom, forming a tetrahedron shape.
How Kunduz Can Help You Learn Lewis Structure of Methane (CH4)?
Understanding the Lewis structure and molecular geometry of methane (CH4) is essential in chemistry. At Kunduz, our experienced tutors will guide you through the process of drawing Lewis structures, understanding molecular geometries, and exploring the properties of different molecules. With Kunduz, you can confidently navigate the world of chemistry and excel in your studies.
Related Topics: