Lewis structures, also known as Lewis dot structures or electron dot structures, are diagrams that represent the arrangement of valence electrons in a molecule or ion. These structures are named after Gilbert N. Lewis, who introduced the concept in 1916. Lewis structures play a crucial role in understanding the bonding and geometry of molecules.
In this article, we will focus on the Lewis structure of H2O, commonly known as water. Water is a vital compound that exists in abundance on Earth and is essential for life as we know it. Understanding the Lewis structure of water will help us comprehend its molecular geometry, hybridization, polarity, and other important properties.
What is Hydrogen?
Before we dive into the Lewis structure of water, let’s briefly discuss the elements that make up this molecule. Hydrogen is the lightest element in the periodic table with the atomic number 1. It has one electron and is located in group 1 of the periodic table. Hydrogen is a highly reactive element and readily forms bonds with other elements to achieve a stable electron configuration.
What is Oxygen?
Oxygen is a chemical element with the atomic number 8 and is a member of group 16 in the periodic table. It is a highly abundant element and makes up about 21% of the Earth’s atmosphere. Oxygen is a vital component for many biological processes, including respiration. It has six valence electrons and can form stable bonds with other elements.
What are the Valence Electrons?
Valence electrons are the outermost electrons of an atom that participate in chemical bonding. They are crucial in determining the chemical properties and reactivity of elements. For hydrogen, the valence electron configuration is 1s1, meaning it has one valence electron. Oxygen, on the other hand, has the valence electron configuration 2s2 2p4, with six valence electrons.
Lewis Structure of Water (H2O)
Now that we have an understanding of the elements involved, let’s explore the Lewis structure of water (H2O). Water consists of two hydrogen atoms bonded to a central oxygen atom. The Lewis structure of water reveals the arrangement of valence electrons in the molecule.
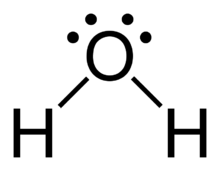
To draw the Lewis structure of water, we follow a step-by-step process:
Step 1: Determine The Total Number Of Electrons In The Valence Shells Of Hydrogen And Oxygen Atoms
To determine the total number of valence electrons in the H2O molecule, we add up the valence electrons of hydrogen and oxygen. Hydrogen has one valence electron, and since there are two hydrogen atoms, the total valence electrons contributed by hydrogen is 2 (1 x 2). Oxygen has six valence electrons. Therefore, the total valence electrons in the H2O molecule is 8 (2 + 6).
Step 2: Total Electron Pairs As Lone Pairs And Bonds
Next, we calculate the total number of electron pairs in the H2O molecule. This includes both lone pairs and bonded pairs of electrons. In the case of water, there are four electron pairs in the valence shells of the atoms.
Step 3: Choosing The Central Atom
In the Lewis structure of water, it is important to determine the central atom. The central atom is usually the atom with the highest valence. In the case of water, oxygen is the central atom since it has a higher valence than hydrogen.
Step 4: Mark Lone Pairs On Atoms
After identifying the central atom, we mark the lone pairs on the atoms. In the Lewis structure of water, oxygen has two lone pairs of electrons since it has six valence electrons. Hydrogen, being an outer atom, cannot accommodate more than two electrons in its valence shell, so it does not have any lone pairs.
Step 5: If There Are Charges On Atoms, Mark Them
In the case of water, there are no charges on the oxygen atom or the hydrogen atoms.
Step 6: To Obtain The Best Lewis Structure Minimize Charges On Atoms By Converting Lone Pairs To Bonds
To ensure the best Lewis structure for water, we check the stability of each atom. If there are charges on the atoms, we aim to minimize them by converting lone pairs to bonds. However, in the case of water, there are no charges on the atoms, so we have already obtained the best Lewis structure.
By following these steps, we can draw the Lewis structure of water, which shows the arrangement of valence electrons and the bonding between atoms. In the Lewis structure of water, there are two single bonds between the oxygen atom and each hydrogen atom. Additionally, there are two lone pairs on the oxygen atom.
The Lewis structure of water can be represented as:
H:O:H
Molecular Geometry of H2O
The Lewis structure of water provides valuable information about its molecular geometry. Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule and is influenced by the bonding and lone pairs of electrons.
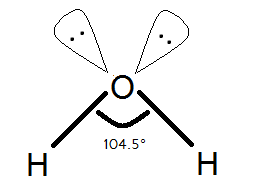
In the case of water, the molecular geometry is bent or V-shaped. This geometry arises due to the presence of two lone pairs of electrons on the oxygen atom. The presence of these lone pairs affects the arrangement of the hydrogen atoms, causing the molecule to adopt a bent shape.
According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, the lone pairs of electrons exert a stronger repulsion compared to the bonding pairs. This repulsion causes the hydrogen atoms to be pushed closer to each other, resulting in a smaller bond angle.
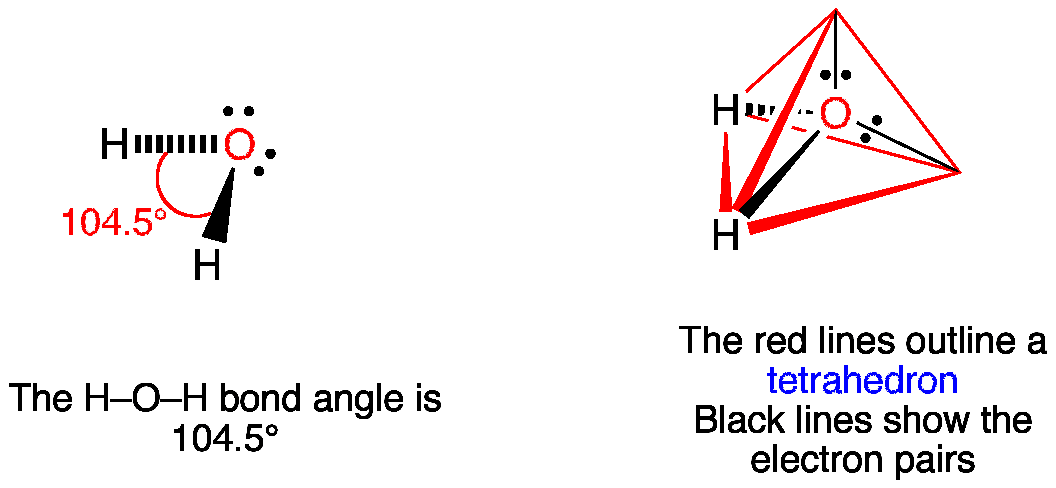
The bond angle in water is approximately 104.5 degrees, deviating slightly from the ideal tetrahedral angle of 109.5 degrees. This deviation is due to the repulsion between the lone pairs of electrons and the bonding pairs.
The bent molecular geometry of water plays a significant role in its physical and chemical properties. It influences the molecule’s polarity, dipole moment, and interactions with other molecules.
Molecular Orbital Diagram of Water (H2O)
To understand the electronic structure of water, we can examine its molecular orbital diagram. Molecular orbitals are formed by the combination of atomic orbitals of the constituent atoms. The molecular orbital diagram provides insights into the stability and bonding in a molecule.
In the case of water, the molecular orbital diagram shows the formation of two sigma (σ) bonds between the oxygen atom and the hydrogen atoms. These sigma bonds result from the overlap of the oxygen’s 2p orbitals with the hydrogen’s 1s orbitals.
In addition to the sigma bonds, water also exhibits two lone pairs of electrons on the oxygen atom. These lone pairs occupy the oxygen’s non-bonding molecular orbitals.
The molecular orbital diagram of water illustrates the distribution of electrons and helps in understanding the bonding and stability of the molecule.
Hybridization in H2O
Hybridization is a concept that explains the formation of new orbitals by the mixing of atomic orbitals. In the case of water, the oxygen atom undergoes sp3 hybridization to form four sp3 hybrid orbitals.
During hybridization, one 2s orbital and three 2p orbitals of oxygen combine to form four sp3 hybrid orbitals. These hybrid orbitals are arranged in a tetrahedral geometry around the oxygen atom, with a bond angle of approximately 109.5 degrees.
Three of the sp3 hybrid orbitals overlap with the 1s orbitals of the hydrogen atoms, resulting in the formation of three sigma (σ) bonds. The remaining sp3 hybrid orbital accommodates the two lone pairs of electrons on the oxygen atom.
Hybridization in water allows for the formation of the four sp3 hybrid orbitals, which are essential for the bonding and geometry of the molecule.
Polarity and Dipole Moment of H2O
Water is a polar molecule due to the presence of polar covalent bonds and its bent molecular geometry. The oxygen atom in water is more electronegative than the hydrogen atoms, resulting in an uneven distribution of electron density.
In the Lewis structure of water, the oxygen atom has a partial negative charge (δ-) due to the higher electronegativity, while the hydrogen atoms have partial positive charges (δ+). This separation of charges creates a dipole moment in the molecule, with the oxygen atom being the negative end and the hydrogen atoms being the positive ends.
The dipole moment of water contributes to its unique properties. It gives water a high boiling point, surface tension, and the ability to form hydrogen bonds. These properties are essential for the role of water as a solvent and its various biological functions.
H2O Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the case of water, the oxygen atom follows the octet rule by sharing electrons with two hydrogen atoms.
The oxygen atom in water initially has six valence electrons. By forming two covalent bonds with hydrogen atoms, it shares two additional electrons, resulting in a total of eight valence electrons. This allows the oxygen atom to achieve a stable electron configuration similar to that of a noble gas.
The octet rule is a fundamental concept in understanding the stability and reactivity of molecules, including water.
H2O Bond Angles
The bond angle in water refers to the angle between the two hydrogen-oxygen bonds. Due to the presence of two lone pairs of electrons on the oxygen atom, the bond angle in water is slightly less than the ideal tetrahedral angle of 109.5 degrees.
Experimental measurements have determined the bond angle in water to be approximately 104.5 degrees. This deviation from the ideal angle is a result of the repulsion between the lone pairs of electrons and the bonding pairs.
The bond angle in water plays a crucial role in the molecule’s molecular geometry and properties. It affects the shape of the molecule and influences its physical and chemical behavior.
Resonance Structure of H2O
Resonance structures are alternative Lewis structures that represent the delocalization of electrons in a molecule or ion. In the case of water, there are no significant resonance structures due to the absence of multiple bonds or delocalized electrons.
However, it is important to note that the concept of resonance can be applied to molecules or ions that possess multiple bonds or electron delocalization.
Geometrical Structure of the Water (H2O)
The geometrical structure of water refers to its three-dimensional arrangement in space. As mentioned earlier, the water molecule adopts a bent or V-shaped geometry due to the presence of two lone pairs of electrons on the oxygen atom.
The bent geometry results in a non-linear arrangement of the hydrogen atoms around the oxygen atom. This molecular geometry is a consequence of the repulsion between the lone pairs and the bonding pairs of electrons.
The geometrical structure of water is crucial in understanding its properties, including its dipole moment, polarity, and intermolecular interactions.
Solved Examples on Lewis Structure of Water (H2O)
Let’s solve some examples to further solidify our understanding of the Lewis structure of water.
Example 1: Draw the Lewis structure of water (H2O).
Solution: To draw the Lewis structure of water, we follow the step-by-step process outlined earlier. First, we determine the total number of valence electrons. Oxygen has 6 valence electrons, and each hydrogen atom has 1 valence electron. Thus, the total valence electrons in water are 8 (6 + 1 + 1).
Next, we identify the central atom, which is oxygen. Hydrogen can only form one bond, so it is placed on the outside of the oxygen atom. We draw the two O-H bonds and mark the two lone pairs of electrons on the oxygen atom.
The resulting Lewis structure of water is:
H:O:H
Example 2: Determine the molecular geometry and bond angle in water.
Solution: In water, the molecular geometry is bent or V-shaped. The bond angle in water is approximately 104.5 degrees, slightly less than the ideal tetrahedral angle of 109.5 degrees.
Example 3: Is water a polar molecule?
Solution: Yes, water is a polar molecule. The oxygen atom in water is more electronegative than the hydrogen atoms, resulting in an uneven distribution of electron density. This creates a dipole moment, with the oxygen atom having a partial negative charge and the hydrogen atoms having partial positive charges.
How Kunduz Can Help You Learn Lewis Structure of Water (H2O)?
At Kunduz, we understand the importance of mastering concepts like Lewis structures. Through our user-friendly interface, our expert educators are dedicated to providing clear and concise explanations, ensuring that you grasp the fundamentals of chemistry.
Whether you are a high school student preparing for exams or a college student pursuing a degree in chemistry, Kunduz is here to support your learning journey. With our affordable and accessible platform, you can study at your own pace and gain a solid foundation in chemistry.
Visit Kunduz today and unlock the power of online learning to master the Lewis structure of water and other essential concepts in chemistry.
Related Topics:
Related Topics:
