Chemical formulas are essential tools in the field of chemistry. They provide a concise and symbolic representation of the elements present in a compound and the ratios in which these elements combine. Understanding chemical formulas is crucial for scientists to communicate and analyze chemical compositions accurately. In this article, we will explore the definition, types, and examples of chemical formulas.
An Introduction to Chemical Formula
Chemistry is the branch of science that investigates the properties and behavior of substances, leading to the formation of new compounds. Chemical formulas serve as a method for scientists to represent the composition of these compounds. By utilizing chemical elements, their symbols, and numerical subscripts, chemical formulas convey the number and type of atoms present in a molecule or compound.
Chemical formulas are concise and standardized, allowing scientists to communicate complex chemical compositions efficiently. They play a crucial role in various aspects of chemistry, including chemical equations, molecular structures, and empirical analysis.
What is a Chemical Formula?
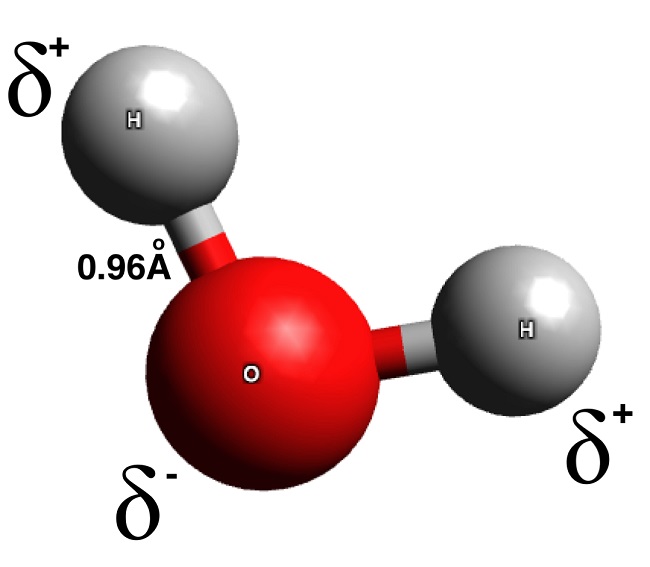
A chemical formula is an expression that represents the elements and their proportions in a compound or molecule. It consists of the chemical symbols of the elements involved, along with numerical subscripts indicating the number of atoms of each element present. For example, the chemical formula for water is H2O, indicating that there are two hydrogen atoms (H) and one oxygen atom (O) in each water molecule.
Chemical formulas provide valuable information about the composition of compounds. They allow scientists to determine the elements present in a substance and the ratios in which they combine. Chemical formulas are essential for identifying and classifying compounds, as well as predicting their chemical properties and reactions.
Importance of Chemical Formula
Chemical formulas play a crucial role in understanding and analyzing chemical compounds. Here are some key reasons why chemical formulas are important:
- Chemical Composition: Chemical formulas provide insight into the elements present in a compound. They allow scientists to identify the types of atoms that make up a substance.
- Ratio of Elements: Chemical formulas represent the ratios in which the constituent elements combine to form a compound. This information is vital for understanding the structure and properties of compounds.
- Chemical Equations: The chemical formula of a compound is essential when representing it in a chemical equation. It helps balance the equation and accurately depict the reactants and products involved.
- Representation of Ions and Free Radicals: Chemical formulas can also be used to represent ions, free radicals, and other chemical species. They provide a concise way to describe these entities and their charges.
Understanding chemical formulas is fundamental for chemists and researchers working in various scientific disciplines. They serve as a universal language that enables effective communication and analysis of chemical compounds.
Types of Chemical Formula
While the term “chemical formula” typically refers to the molecular formula of a compound, which denotes the total number of atoms of each constituent element in one molecule of the compound, the compositions of chemical compounds can be expressed in several ways. Let’s explore the different types of chemical formulas:
Molecular Formula
The molecular formula provides insight into the number of elements present in a compound. In molecular formulas, the elements are denoted by their respective symbols from the periodic table, and the number of atoms of each element in the molecule is written as a subscript. For example, the molecular formula for glucose is C6H12O6, indicating that each molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Molecular formulas give a precise representation of the composition of a compound. They are particularly useful for understanding the stoichiometry of reactions and determining the molecular weight of a substance.
Empirical Formula
The empirical formula represents the simplest ratio of the elements present in a compound. It is usually obtained based on experimental data and does not provide the exact number of atoms in a molecule. The empirical formula can be derived from the molecular formula by dividing all the subscripts by their greatest common divisor.
For example, the molecular formula of glucose is C6H12O6. By dividing all the subscripts by 6 (the greatest common divisor), we obtain the empirical formula CH2O. This means that the ratio of carbon to hydrogen to oxygen in glucose is 1:2:1.
Empirical formulas are valuable for understanding the fundamental composition of compounds. They provide a simplified representation of the molecular structure and allow for comparisons between different compounds.

Structural Formula
The structural formula provides detailed information about the arrangement of atoms in a molecule. It shows how the atoms are bonded to each other and the connectivity between them. Structural formulas are often represented using lines to indicate chemical bonds and show the spatial orientation of atoms.
For example, the structural formula of glucose is:
H H H
| | |
H - C - C - C - C - O - H
| | |
H OH OH
In this representation, each line represents a chemical bond, and the atoms are arranged in a specific order. Structural formulas are especially useful for understanding the three-dimensional structure of molecules and predicting their properties and reactivity.
How to Write Chemical Formula
Writing a chemical formula involves knowing the symbols of the elements present in the compound, the formulas of radicals, and the valency of the elements. Here are some key points to keep in mind when writing a chemical formula:
- Most compounds are binary compounds, meaning they consist of two elements. However, compounds with more than two elements are also known.
- An atom with a positive charge is called a cation, while an atom with a negative charge is called an anion.
- When a compound contains both a metal and a non-metal, the metal is named first, followed by the non-metal. For example, NaCl consists of Na+ (sodium ion) and Cl- (chloride ion).
- Anions with a -1 negative charge usually have the suffix “-ide.” For example, F- is fluoride.
- Anions with oxyanions (oxygen + another element) usually have the suffix “-ate.” For example, SO42- is sulfate.
- Some polyatomic anions have specific names, such as NH2- (amide), PO43- (phosphate), and CN- (cyanide).
Writing chemical formulas requires knowledge of the valency of elements and their charges. By following the rules mentioned above, chemists can accurately represent the composition of compounds.
List of Chemical Compound Formulas
Chemistry encompasses a wide range of compounds with various chemical formulas. Here is a list of important chemical compound formulas:
| Sl.No | Chemical Compound | Chemical Formula |
| 1 | Acetic acid | CH3COOH |
| 2 | Aluminium hydroxide | Al(OH)3 |
| 3 | Acetate | CH3COO¯ |
| 4 | Acetone | C3H₆O |
| 5 | Aluminium acetate | C₆H₉AlO₆ |
| 6 | Aluminium bromide | AlBr3 |
| 7 | Aluminium carbonate | Al2(CO3)3 |
| 8 | Aluminium chloride | AlCl3 |
| 9 | Aluminium fluoride | AlF3 |
| 10 | Aluminium | Al |
| 11 | Aluminium iodide | AlI3 |
| 12 | Aluminium oxide | Al2O3 |
| 13 | Aluminium phosphate | AlPO₄ |
| 14 | Amino acid | H2NCHRCOOH |
| 15 | Ammonia | NH₄ |
| 16 | Ammonium dichromate | Cr2H₈N2O₇ |
| 17 | Ammonium acetate | C2H3O2NH₄ |
| 18 | Ammonium bicarbonate | NH₄HCO3 |
| 19 | Ammonium bromide | NH₄Br |
| 20 | Ammonium carbonate | (NH₄)2CO3 |
| 21 | Ammonium chloride | NH₄Cl |
| 22 | Ammonium hydroxide | NH₄OH |
| 23 | Ammonium iodide | NH₄I |
| 24 | Ammonium nitrate | NH₄NO3 |
| 25 | Aluminium sulfide | Al2S3 |
| 26 | Ammonium nitrite | NH₄NO2 |
| 27 | Ammonium oxide | (NH₄)2O |
| 28 | Ammonium phosphate | (NH₄)3PO₄ |
| 29 | Ammonium sulfate | (NH₄)2SO₄ |
| 30 | Ammonium sulfide | (NH₄)2S |
| 31 | Argon gas | Ar |
| 32 | Ascorbic acid | C₆H₈O₆ |
| 33 | Barium acetate | Ba(C₂H3O2)2 |
| 34 | Barium bromide | BaBr2 |
| 35 | Barium chloride | BaCl2 |
| 36 | Barium fluoride | BaF2 |
| 37 | Barium hydroxide | Ba(OH)2 |
| 38 | Barium iodide | BaI2 |
| 39 | Barium nitrate | Ba(NO3)2 |
| 40 | Barium oxide | BaO |
| 41 | Barium phosphate | Ba3O₈P2 |
| 42 | Barium sulfate | BaSO₄ |
| 43 | Benzene | C₆H₆ |
| 44 | Benzoic acid | C₇H₆O2 |
| 45 | Bicarbonate | CHO3¯ |
| 46 | Bleach | NaClO |
| 47 | Boric acid | H3BO3 |
| 48 | Potassium Bromate | KBrO3 |
| 49 | Bromic acid | HBrO3 |
| 50 | Bromine | Br |
| 51 | Butane | C₄H₁₀ |
| 52 | Butanoic acid | C₄H₈O2 |
| 53 | Calcium acetate | C₄H₆CaO₄ |
| 54 | Calcium bromide | CaBr2 |
| 55 | Calcium carbonate | CaCO3 |
| 56 | Calcium hydride | CaH2 |
| 57 | Calcium hydroxide | Ca(OH)2 |
| 58 | Calcium iodide | CaI2 |
| 59 | Calcium nitrate | Ca(NO3)2 |
| 60 | Calcium oxide | CaO |
| 61 | Carbon monoxide | CO |
| 62 | Carbon tetrachloride | CCl₄ |
| 63 | Carbonic acid | H2CO3 |
| 64 | Calcium phosphate | Ca3(PO₄)2 |
| 66 | Citric acid | C₆H₈O₇ |
| 67 | Chlorate | ClO3¯ |
| 68 | Chlorine | Cl |
| 69 | Chlorine gas | Cl2 |
| 70 | Chlorous acid | HClO2 |
| 71 | Chromate | CrO₄²¯ |
| 72 | Chromic acid | H2CrO₄ |
| 73 | Citric acid | C₆H₈O₇ |
| 74 | Copper ii carbonate | CuCO3 |
| 75 | Copper ii nitrate | Cu(NO3)2 |
| 76 | Cyanide | CN¯ |
| 77 | Dichromate | K2Cr2O₇ |
| 78 | Dihydrogen monoxide | OH2 |
| 79 | Dinitrogen monoxide | N2O |
| 80 | Dinitrogen pentoxide | N2O₅ |
| 81 | Dinitrogen trioxide | N2O3 |
| 82 | Ethanol | C2H₅OH |
| 83 | Iron oxide | Fe2O3 |
| 84 | Ethylene glycol | C2H₆O2 |
| 85 | Fluorine gas | F2 |
| 86 | Aluminium bromide | AlBr3 |
| 87 | Aluminium sulphide | Al2S3 |
| 88 | Ammonium carbonate | (NH₄)2CO3 |
| 89 | Ammonium nitrate | (NH₄)(NO3) |
| 90 | Ammonium phosphate | (NH₄)3PO₄ |
| 91 | Barium chloride | BaCl2 |
| 92 | Barium sulphate | BaSO₄ |
| 93 | Calcium nitrate | Ca(NO3)2 |
| 94 | Carbon monoxide | CO |
| 95 | Carbon tetrachloride | CCl₄ |
| 96 | Carbonic acid | H2CO3 |
| 97 | Hydrofluoric acid | HF |
| 98 | Hydroiodic acid | HI |
| 99 | Hypochlorous acid | HClO |
| 100 | Lithium phosphate | Li3PO₄ |
| 101 | Magnesium nitrate | MgNO3 |
| 102 | Magnesium phosphate | Mg3(PO₄)2 |
| 103 | Nitrogen monoxide | NO |
| 104 | Nitrous acid | HNO2 |
| 105 | Potassium carbonate | K2CO3 |
| 106 | Potassium iodide | KI |
| 107 | Potassium nitrate | KNO3 |
| 108 | Potassium phosphate | KH2PO₄ |
| 109 | Sodium carbonate | Na2CO₄ |
| 110 | Sodium oxide | Na2O |
| 111 | Fructose chemical | C₆H₁2O₆ |
| 112 | Glycerol | C3H₈O3 |
| 113 | Helium gas | He |
| 114 | Hexane | C₆H₁₄ |
| 115 | Hydrobromic acid | HBr |
| 116 | Hydrochloric acid | HCl |
| 117 | Hydrocyanic acid | HCN |
| 118 | Hydrofluoric acid | HF |
| 119 | Hydrogen carbonate | CHO3¯ |
| 120 | Hydrogen gas | H2 |
| 121 | Hydrogen peroxide | H2O2 |
| 122 | Hydrogen phosphate | H3PO₄ |
| 123 | Hydrogen sulphate | HSO₄¯ |
| 124 | Hydroiodic acid | HI |
| 125 | Hydrosulfuric acid | H2SO₄ |
| 126 | Hydroxide | OH¯ |
| 127 | Hypobromous acid | HBrO |
| 128 | Hypochlorite | NaClO |
| 129 | Hypochlorous acid | HClO |
| 130 | Hypoiodous acid | HIO |
| 131 | Iodic acid | HIO3 |
| 132 | Iodide | I¯ |
| 133 | Iodine | I₂ |
| 134 | Iron iii nitrate | Fe(NO3)3 |
| 135 | Iron ii oxide | FeO |
| 136 | Iron iii carbonate | Fe2(CO3)3 |
| 137 | Iron iii hydroxide | Fe(OH)3 |
| 138 | Iron iii oxide | Fe2O3 |
| 139 | Iron iii chloride | FeCl3 |
| 140 | Lactic acid | C3H6O3 |
| 141 | Lead acetate | Pb(C2H3O2)2 |
| 142 | Lead ii acetate | Pb(C2H3O2)2 |
| 143 | Lead iodide | PbI2 |
| 144 | Lead iv oxide | PbO2 |
| 145 | Lead nitrate | Pb(NO3)2 |
| 146 | Lithium bromide | LiBr |
| 147 | Lithium chloride | LiCl2 |
| 148 | Lithium hydroxide | LiOH |
| 149 | Lithium iodide | LiI |
| 150 | Lithium oxide | Li2O |
| 151 | Lithium phosphate | Li3PO4 |
| 152 | Magnesium acetate | Mg(CH3COO)2 |
| 153 | Magnesium bicarbonate | C2H2MgO6 |
| 154 | Magnesium carbonate | MgCO3 |
| 155 | Magnesium chloride | MgCl2 |
| 156 | Magnesium hydroxide | Mg(OH)2 |
| 157 | Magnesium iodide | MgI2 |
| 158 | Magnesium nitrate | Mg(NO3)2 |
| 159 | Magnesium nitride | Mg3N2 |
| 160 | Magnesium carbonate | MgCO3 |
| 161 | Magnesium bromide | MgBr2 |
| 162 | Magnesium oxide | MgO |
| 163 | Magnesium phosphate | Mg3(PO4)2 |
| 164 | Magnesium sulphate | MgSO4 |
| 165 | Magnesium sulphide | MgS |
| 166 | Methane | CH4 |
| 167 | Methanol | CH3OH |
| 168 | Nickel acetate | Ni(C2H3O2)2 |
| 169 | Nickel nitrate | Ni(NO3)2 |
| 170 | Nitric acid | HNO3 |
| 171 | Nitride | N3– |
| 172 | Nitrite | NO2− |
| 173 | Nitrogen dioxide | NO2 |
| 174 | Nitrogen monoxide | NO |
| 175 | Nitrous acid | HNO2 |
| 176 | Oxalate | C2O42¯ |
| 177 | Oxalic acid | H2C2O4 |
| 178 | Oxygen | O2 |
| 179 | Ozone | O3 |
| 180 | Perbromic acid | HBrO4 |
| 181 | Potassium Permanganate | KMnO4 |
| 182 | Permanganate ion | MnO4– |
| 183 | Phosphate | PO43- |
| 184 | Sodium hydrogen phosphate | Na2HPO4 |
| 185 | Sodium formate | CHNaO2 |
| 186 | Phosphoric acid | H3PO4 |
| 187 | Phosphorus pentachloride | PCl5 |
| 188 | Phosphorus trichloride | PCl3 |
| 189 | Potassium acetate | CH3CO2K |
| 190 | Potassium bicarbonate | KHCO3 |
| 191 | Potassium carbonate | K2CO3 |
| 192 | Potassium chlorate | KClO3 |
| 193 | Potassium hydrogen phosphate | K2HPO4 |
| 194 | Potassium chloride | KCl |
| 195 | Potassium chromate | CrK2O4 |
| 196 | Potassium cyanide | KCN |
| 197 | Potassium dichromate | K2Cr2O7 |
| 198 | Potassium fluoride | KF |
| 199 | Potassium hydroxide | KOH |
| 200 | Potassium hypochlorite | KClO |
Molecular Mass from Chemical Formula
The molecular mass, also known as the molar mass, of a compound can be calculated using its chemical formula. The molecular mass is the sum of the atomic masses of all the atoms in the compound.
To determine the molecular mass, multiply the atomic mass of each element by the number of atoms present in the compound, as indicated by the subscripts in the chemical formula. Then, sum up the masses of all the elements to obtain the molecular mass.
For example, let’s calculate the molecular mass of glucose (C6H12O6):
- Carbon (C) atomic mass = 12.01 g/mol
- Hydrogen (H) atomic mass = 1.01 g/mol
- Oxygen (O) atomic mass = 16.00 g/mol
Molecular mass of glucose = (6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
The molecular mass provides crucial information for stoichiometry calculations and determining the amount of substance in a given sample.
Solved Examples on Chemical Formula
Let’s explore some examples to solidify our understanding of chemical formulas:
Example 1: Sodium Chloride (NaCl)
In one molecule of sodium chloride, determine how many atoms of each element are present.
Answer: There is one atom of sodium (Na) and one atom of chlorine (Cl) in each molecule of sodium chloride.
Example 2: Glucose (C6H12O6)
In one molecule of glucose, determine how many atoms of each element are present.
Answer: In one molecule of glucose, there are six carbon atoms (C), twelve hydrogen atoms (H), and six oxygen atoms (O).
Example 3: Hydroxyapatite (Ca10(PO4)6(OH)2)
In one molecule of hydroxyapatite, determine how many atoms of each element are present.
Answer: There are ten calcium atoms (Ca), six phosphate ions (PO43-), and two hydroxide ions (OH-) in each molecule of hydroxyapatite.
These examples demonstrate how chemical formulas provide valuable information about the composition of compounds and the number of atoms of each element present.
How Kunduz Can Help You Learn Chemical Formula?
Kunduz is a comprehensive online learning platform that offers a wide range of educational resources for students studying chemistry. With Kunduz, you can access interactive lessons, practice problems, and detailed explanations to enhance your understanding of chemical formulas.
Kunduz provides step-by-step guidance on writing chemical formulas, understanding molecular and empirical formulas, and interpreting structural formulas. Our expert instructors are available to answer any questions you may have and provide personalized assistance to help you master the concepts.
Whether you’re a beginner or an advanced chemistry student, Kunduz offers a supportive and engaging learning environment. With our user-friendly interface and comprehensive resources, you can develop a strong foundation in chemistry and excel in your academic pursuits.
