Dipole moment is a fundamental concept in chemistry that describes the separation of charges in a system. It is commonly observed in both ionic and covalent compounds, and is a measure of the polarity of a molecule. In this article, we will explore the definition, types, formula, examples, and frequently asked questions about dipole moment.
For readers exploring the concept of dipole moments and interested in broader chemistry topics, our benzoic acid and Boltzmann constant pages serve as valuable references. These resources offer insights into the chemical properties of benzoic acid and the fundamental role of the Boltzmann constant, providing a well-rounded understanding that complements the exploration of dipole moments within the broader context of chemical principles.
An Introduction to the Dipole Moment
When atoms form chemical bonds, they share electrons unequally due to differences in electronegativity. This unequal sharing of electrons creates a separation of charges within the molecule, resulting in a dipole moment. The dipole moment is a vector quantity, meaning it has both magnitude and direction.
A dipole moment can occur between two ions in an ionic bond or between atoms in a covalent bond. The magnitude of the dipole moment depends on the difference in electronegativity between the atoms involved in the bond. The larger the difference in electronegativity, the larger the dipole moment.
What is a Dipole Moment?
A dipole moment is a measure of the polarity of a molecule. It is defined as the product of the charge and the distance between the charges. Mathematically, the dipole moment (μ) can be calculated using the formula:
μ = q * d
Where μ is the dipole moment, q is the magnitude of the charge, and d is the distance between the charges. The unit of dipole moment is the Debye (D), where 1 Debye is equal to 3.33564 × 10^-30 C.m.
The dipole moment is a vector quantity, meaning it has both magnitude and direction. The direction of the dipole moment is from the positive charge to the negative charge.

Dipole Moment Formula
The dipole moment can be calculated using the formula:
μ = q * d
Where μ is the dipole moment, q is the magnitude of the charge, and d is the distance between the charges.
Unit and Dimensions of Dipole Moment
The dipole moment is measured in Debye units, denoted by D. 1 Debye is equal to 3.33564 × 10^-30 C.m, where C is Coulomb and m denotes a meter. The dimensional formula of dipole moment is [M^0L^1T^1I^1].
Dipole Moment Equation
The dipole moment of a molecule can be calculated using the equation:
μ = ∑q * r
Where μ is the dipole moment vector, q_i is the magnitude of the i-th charge, and r_i is the vector representing the position of the i-th charge.
The dipole moment acts in the direction of the vector quantity. For example, in a water molecule (H2O), the presence of a lone pair on the oxygen atom causes the molecule to have a bent shape. As a result, the dipole moments of the individual O-H bonds do not cancel each other out, making water a polar molecule.
Polarity and Structure of Molecules
The shape of a molecule and the polarity of its bonds determine the overall polarity of that molecule. A molecule that contains polar bonds may not have an overall polarity if its shape is symmetrical. In order for a molecule to be polar, its structure must be asymmetrical.
The polarity of a bond depends on the difference in electronegativity between the two atoms involved. The larger the difference in electronegativity, the more polar the bond. The dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together.
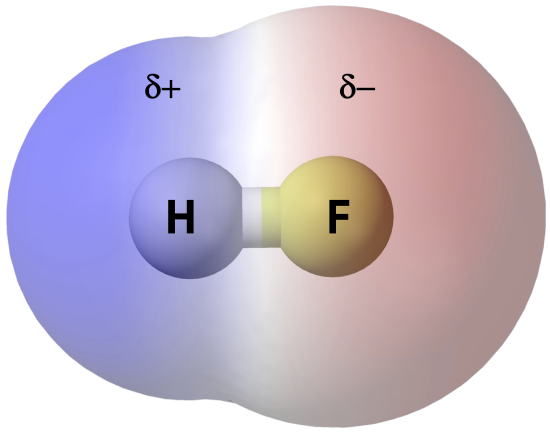
Polar Molecules and Dipole-Dipole Interaction
Polar molecules have a net dipole moment due to the separation of charges within the molecule. This dipole moment leads to dipole-dipole interactions between molecules. Dipole-dipole interactions are attractive forces that occur between the positive end of one polar molecule and the negative end of another polar molecule.
These interactions are stronger than London dispersion forces, which are the attractive forces between nonpolar molecules. The strength of dipole-dipole interactions depends on the magnitude of the dipole moment and the distance between the molecules.
Polar Molecules and Ions Interaction
In addition to dipole-dipole interactions, polar molecules can also interact with ions. The positive end of a polar molecule is attracted to negatively charged ions, while the negative end is attracted to positively charged ions. These interactions are important in many chemical reactions and play a significant role in determining the properties of compounds.
Significance of Electric Dipole Moment
The electric dipole moment is a fundamental property of molecules that influences their physical and chemical properties. It is used to determine the polarity of a compound, which affects its solubility, boiling point, and melting point. The dipole moment also plays a role in determining the reactivity and intermolecular forces of a molecule.
Understanding the dipole moment of a molecule is important in fields such as organic chemistry, materials science, and biochemistry. It provides insights into the structure, properties, and behavior of compounds, and is a key factor in understanding molecular interactions.
Electric Dipole Moment
The electric dipole moment is a measure of the separation of positive and negative charges in a system. It is a vector quantity, meaning it has both magnitude and direction. The direction of the dipole moment is from the positive charge to the negative charge.
The electric dipole moment is represented by an arrow symbol, with a cross on the positive center and an arrowhead on the negative center. This symbolizes the shift of electron density in the molecule.
Examples of Dipole Moment
Let’s consider some examples of dipole moments in different molecules.
Dipole moment of BeF2
In a beryllium fluoride (BeF2) molecule, the bond angle between the two Be-F bonds is 180 degrees, resulting in a linear structure. The two individual bond dipole moments in BeF2 cancel each other out because they are equal in magnitude but opposite in direction. Therefore, the net dipole moment of a BeF2 molecule is zero.
Dipole moment of H2O (Water)
In a water (H2O) molecule, the oxygen atom is more electronegative than the hydrogen atoms, resulting in a polar covalent bond. The presence of two lone pairs of electrons on the oxygen atom gives the water molecule a bent shape. Due to the bent structure and the unequal distribution of charge, the individual bond dipole moments in water do not cancel each other out. As a result, water has a net dipole moment of 1.85 D.
Dipole Moment of CO2
In a carbon dioxide (CO2) molecule, the carbon-oxygen double bonds are polar, but the molecule as a whole is nonpolar. This is because the molecule has a linear structure, and the individual bond dipole moments cancel each other out due to their equal magnitude but opposite direction. Therefore, the net dipole moment of a CO2 molecule is zero.
Dipole moment in BH3 and NH3
In a boron trihydride (BH3) molecule, the three B-H bonds are symmetrically arranged in a plane, resulting in a net dipole moment of zero. On the other hand, ammonia (NH3) has a pyramidal structure, with three N-H bonds and a lone pair on the nitrogen atom. This asymmetrical arrangement gives the NH3 molecule a net dipole moment of 1.49 D.
Dipoles in an External Electric Field
When an electric dipole is placed in an external electric field, it experiences a torque. The torque on the dipole is maximum when it is perpendicular to the electric field and minimum when it is parallel to the electric field.
Uses of Dipole Moment
The dipole moment has various uses in chemistry. It helps to determine the symmetry of molecules, distinguish between cis- and trans-isomers, and calculate the percentage ionic character of a molecule. The dipole moment is also used to study the behavior of molecules in an electric field and to classify compounds based on their polar or nonpolar nature.
Frequently Asked Questions on Dipole Moment
Where Do Dipole Moments Occur?
Dipole moments occur in both ionic and covalent bonds. They arise from the differences in electronegativity between the atoms involved in the bond. The larger the difference in electronegativity, the larger the dipole moment.
What is the Dipole Moment and Its SI Unit?
The dipole moment is a measure of the separation of negative and positive charges within a system or molecule. It is a vector quantity that has both a direction and a magnitude. The SI unit for the dipole moment is the Debye (D).
What Causes a Dipole Moment in a Molecule?
A dipole moment in a molecule is caused by the unequal sharing of electrons between the atoms involved in a bond. This unequal sharing is due to differences in electronegativity between the atoms. The more electronegative atom pulls the shared electrons towards itself, creating a partial negative charge, while the less electronegative atom has a partial positive charge.
How is a Dipole Moment Calculated?
The dipole moment is calculated as the product of the magnitude of the charge and the distance between the charges. The formula for the dipole moment is μ = Q * d, where μ is the dipole moment, Q is the magnitude of the charge, and d is the distance between the charges.
What Does It Mean If a Molecule Has a Zero Dipole Moment?
If a molecule has a zero dipole moment, it means that the molecule is nonpolar. This is usually because the molecule has a symmetrical structure, causing the individual bond dipole moments to cancel each other out.
Why is the Dipole Moment Important?
The dipole moment is important because it provides information about the charge distribution in a molecule. This can help in predicting the molecule’s behavior in various chemical reactions. It also helps in determining the polarity of a bond, the symmetry of a molecule, and the percentage of ionic character in a molecule.
When is the Torque on a Dipole Maximum?
The torque on a dipole is maximum when the dipole is oriented perpendicular to the electric field. This is because the torque depends on the angle between the dipole moment vector and the electric field vector.
When is the Torque on a Dipole Minimum?
The torque on a dipole is minimum when the dipole is oriented parallel or antiparallel to the electric field. In this orientation, the angle between the dipole moment vector and the electric field vector is either 0 or 180 degrees, making the torque zero.
Solved Examples on Dipole Moment
Let’s solve some examples to further understand dipole moment calculations.
Example 1: Calculate the dipole moment of a molecule with a charge of 2 C and a separation distance of 3 m.
Solution: Given: Charge (q) = 2 C Separation distance (d) = 3 m
Using the formula for dipole moment: μ = q * d μ = 2 C * 3 m μ = 6 C.m
Therefore, the dipole moment of the molecule is 6 C.m.
Example 2: Determine the dipole moment of a molecule with a charge of 5 x 10^-6 C and a separation distance of 2 x 10^-10 m.
Solution: Given: Charge (q) = 5 x 10^-6 C Separation distance (d) = 2 x 10^-10 m
Using the formula for dipole moment: μ = q * d μ = (5 x 10^-6 C) * (2 x 10^-10 m) μ = 10^-15 C.m
Therefore, the dipole moment of the molecule is 10^-15 C.m.
Example 3: A molecule has a net dipole moment of 3.5 D. If the separation distance between the charges is 1.5 x 10^-9 m, what is the magnitude of the charge?
Solution: Given: Dipole moment (μ) = 3.5 D = 3.5 x 10^-30 C.m Separation distance (d) = 1.5 x 10^-9 m
Using the formula for dipole moment: μ = q * d 3.5 x 10^-30 C.m = q * (1.5 x 10^-9 m) q = (3.5 x 10^-30 C.m) / (1.5 x 10^-9 m) q ≈ 2.333 x 10^-21 C
Therefore, the magnitude of the charge is approximately 2.333 x 10^-21 C.
How Kunduz Can Help You Learn Dipole Moment?
Kunduz is a comprehensive learning platform that provides resources and tools to enhance your understanding of chemistry topics such as dipole moment. With interactive lessons, practice questions, and expert guidance, Kunduz can help you master the fundamentals of chemistry and excel in your academic pursuits.
Whether you are preparing for competitive exams like JEE Main and JEE Advanced or simply want to deepen your knowledge of chemistry, Kunduz is your go-to resource for comprehensive and engaging learning materials. Join Kunduz today and unlock your full potential in the world of chemistry.
