Homogeneous mixtures are a fascinating concept in the field of chemistry. They are a type of mixture that exhibits a uniform composition throughout its entire volume. In this comprehensive guide, we will explore the definition, properties, types, and examples of homogeneous mixtures, as well as delve into the comparison between homogeneous and heterogeneous mixtures.
An Introduction to Homogeneous Mixtures
Mixtures are combinations of two or more substances that are physically blended together. In a homogeneous mixture, the components are so thoroughly mixed that they are indistinguishable from one another. This means that every part of the mixture has the same composition and properties. Homogeneous mixtures can exist in various forms, including solids, liquids, gases, and even plasmas.
What is a Homogeneous Mixture?
A homogeneous mixture, also known as a solution, is a type of mixture in which the composition is uniform throughout. This means that the proportions of the components are the same in every part of the mixture. Whether you take a small sample or a large one, the ratio of the substances will remain constant. Homogeneous mixtures can be observed in our everyday lives, from the air we breathe to the beverages we consume.
What is a Heterogeneous Mixture?
In contrast to homogeneous mixtures, heterogeneous mixtures are characterized by non-uniform composition. This means that the components are not evenly distributed throughout the mixture. Heterogeneous mixtures can often be visually identified due to the presence of distinct phases or regions with different properties. Examples of heterogeneous mixtures include oil and water, sand and water, and salad with various ingredients.
:max_bytes(150000):strip_icc()/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)
Homogeneous vs. Heterogeneous Mixtures
The key difference between homogeneous and heterogeneous mixtures lies in their uniformity or lack thereof. Homogeneous mixtures have a consistent composition throughout, whereas heterogeneous mixtures have varying compositions in different parts of the mixture. In a homogeneous mixture, the components are evenly distributed and cannot be visually distinguished. On the other hand, in a heterogeneous mixture, the components are visibly separated and may exhibit distinct layers or sedimentation.
To further understand the concept of homogeneous mixtures, let’s explore their composition, characteristics, properties, and separation techniques.
| Homogeneous Mixtures | Heterogeneous Mixtures |
|---|---|
| Uniform composition throughout | Non-uniform composition |
| Appears as a single phase | Multiple visible phases |
| Examples: Saltwater, Air, Sugar dissolved in water | Examples: Salad with various ingredients, Soil with rocks, Oil and water mixture |
Composition of Homogeneous Mixtures
Homogeneous mixtures are composed of two or more substances that are evenly distributed throughout the entire mixture. The composition of a homogeneous mixture remains the same regardless of the amount of sample taken. This uniform distribution of components is what gives homogeneous mixtures their distinctive properties.
The particles present in a homogeneous mixture can vary depending on the state of matter. In a solid homogeneous mixture, the particles are closely packed and have a fixed volume and shape. Examples of solid homogeneous mixtures include saltwater (salt dissolved in water) and sugar dissolved in tea.
In a liquid homogeneous mixture, the particles are not as tightly packed as in a solid, but they still have a fixed volume. Liquid homogeneous mixtures are commonly observed in solutions like vinegar (acetic acid dissolved in water) and fruit juices.
Lastly, in a gaseous homogeneous mixture, the particles are widely dispersed and have no definite volume or shape. Air, which is composed of various gases like nitrogen, oxygen, and carbon dioxide, is a prime example of a gaseous homogeneous mixture.
Characteristics of Homogeneous Mixtures
Homogeneous mixtures possess several important characteristics that distinguish them from other types of mixtures:
- Uniform Composition: The composition of a homogeneous mixture is consistent throughout its entire volume. This means that the ratio of one substance to another remains constant in any portion of the mixture.
- No Visible Boundaries: When you look at a homogeneous mixture, you cannot visually distinguish the individual components. Unlike in a heterogeneous mixture, where different components are visibly separated, homogeneous mixtures appear as a single phase with a uniform appearance.
- Homogeneity at a Molecular Level: The uniformity in a homogeneous mixture extends down to the molecular or atomic level. The individual particles of the substances are mixed evenly, resulting in a uniform distribution of components.
- Can Be Separated by Chemical Means: Although homogeneous mixtures may appear as a single substance, they can often be separated into their components using chemical techniques such as distillation, evaporation, or filtration. These methods take advantage of the different chemical properties of the substances within the mixture.
- Solubility: Homogeneous mixtures often involve the dissolution of a solute (a substance being dissolved) in a solvent (the dissolving medium). The solubility of the solute in the solvent is an important factor in the formation of homogeneous mixtures.
Properties of Homogeneous Mixtures
Homogeneous mixtures exhibit several properties that are characteristic of their uniform composition:
- Consistent Composition: Homogeneous mixtures have the same composition and proportions of their components throughout. This means that every part of the mixture will have the same ratio of substances.
- No Settling: Unlike some heterogeneous mixtures, homogeneous mixtures do not exhibit settling or separation of components over time. The particles in a homogeneous mixture remain evenly distributed throughout the mixture.
- Clarity and Transparency: Many homogeneous mixtures are clear or transparent, allowing light to pass through them without significant scattering. This is due to the uniform distribution of particles within the mixture.
- Consistent Taste and Properties: Homogeneous mixtures have a consistent taste and exhibit uniform properties throughout. This is because the composition and proportions of the components are the same in every part of the mixture.
- Separability: Although homogeneous mixtures appear as a single substance, they can be separated into their components using appropriate separation techniques. These techniques take advantage of the different physical or chemical properties of the substances within the mixture.
Separation Techniques for Homogeneous Mixtures
Homogeneous mixtures can be separated into their individual components using various separation techniques. These techniques exploit the different physical or chemical properties of the substances present in the mixture. Some common separation techniques for homogeneous mixtures include filtration, distillation, and evaporation.
Filtration
Filtration is a separation technique used to remove solid particles from a liquid or gas mixture. It involves passing the mixture through a filter or a porous material, which traps the solid particles while allowing the liquid or gas to pass through. Filtration is commonly used in everyday life, such as when making coffee using a coffee filter or purifying water using a water filter.
Distillation
Distillation is a separation technique that takes advantage of differences in boiling points to separate the components of a homogeneous mixture. It involves heating the mixture to convert one or more components into a vapor, which is then condensed and collected separately. Distillation is commonly used in the production of alcoholic beverages, the purification of water, and the separation of petroleum products.

Evaporation
Evaporation is a separation technique that involves converting a liquid component of a homogeneous mixture into its vapor state, leaving behind the solid or dissolved substances. It is particularly useful when the liquid component is volatile and easily evaporates at relatively low temperatures. Evaporation is commonly used in the production of salt from saltwater, where the water is evaporated, leaving behind the salt crystals.
These separation techniques enable scientists and researchers to isolate and study the individual components of a homogeneous mixture, leading to a deeper understanding of their properties and behaviors.

Classification of Homogeneous Mixtures
Homogeneous mixtures can be further classified into three main types: suspensions, solutions, and colloids. Each type has its own unique characteristics and properties.
Suspensions
A suspension is a heterogeneous mixture in which solid particles are dispersed in a liquid or gas medium. Unlike solutions and colloids, suspensions have particles that are large enough to be visible to the naked eye. The particles in a suspension can settle over time, resulting in a non-uniform appearance. Examples of suspensions include muddy water, flour mixed in water, and slaked lime for whitewashing.
Solutions
Solutions are homogeneous mixtures in which the particles of the solute (substance being dissolved) are evenly dispersed and mixed with the particles of the solvent (substance doing the dissolving). Solutions can exist in various states, such as solids dissolved in liquids (salt dissolved in water), gases dissolved in liquids (carbon dioxide dissolved in soda water), and liquids dissolved in liquids (alcohol dissolved in water). Solutions play a fundamental role in many aspects of our daily lives, from cooking to medicine.
In a solution, the solute is the component that is dissolved, and the solvent is the component that does the dissolving. The solute is typically present in smaller quantities compared to the solvent. For example, when sugar is dissolved in water, sugar acts as the solute, and water acts as the solvent.
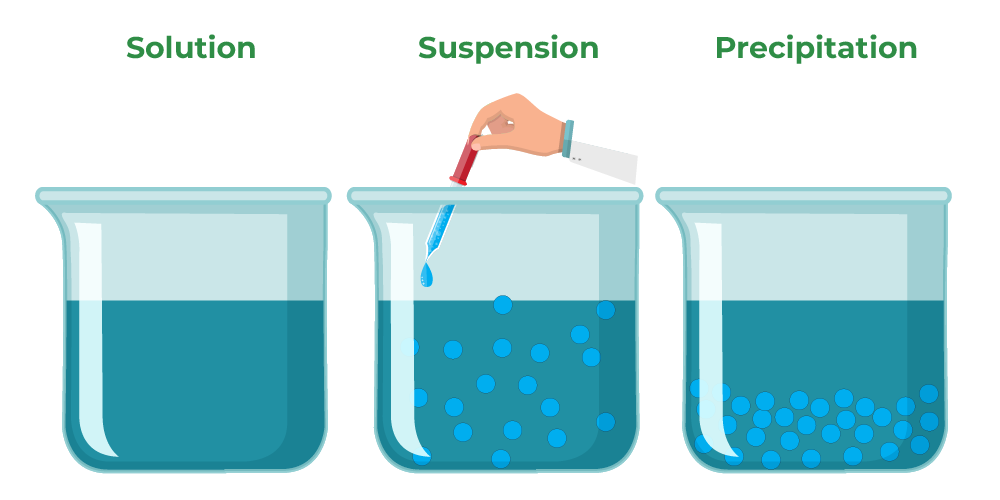
Colloids
Colloids are heterogeneous mixtures in which the particles of the dispersed phase (solid, liquid, or gas) are evenly dispersed within the continuous phase (liquid or solid). Unlike solutions, colloids have particles that are larger than individual molecules but smaller than those in suspensions. This gives colloids unique properties, such as the ability to scatter light.
Colloids can exist in various forms, including sols, emulsions, foams, aerosols, and gels. Examples of colloids include blood (a colloid of cells and plasma), milk (a colloid of fat globules in water), and whipped cream (a colloid of gas bubbles in a liquid).

How to Identify Homogeneous Mixtures?
Identifying homogeneous mixtures can be done through careful observation and understanding of their characteristics. Here are some methods to help you identify homogeneous mixtures:
Visual Inspection
One way to identify a homogeneous mixture is through visual inspection. Homogeneous mixtures appear uniform and do not have visible boundaries or distinct phases. When you look at a homogeneous mixture, you cannot visually distinguish the individual components.
Clarity and Transparency
Many homogeneous mixtures are clear or transparent. If you can see through the mixture without any visible particles or sedimentation, it is likely a homogeneous mixture. Examples of clear and transparent homogeneous mixtures include saltwater, sugar dissolved in water, and clear vinegar.
No Settling
Homogeneous mixtures do not exhibit settling or separation of components over time. Unlike some heterogeneous mixtures that may show distinct layers or sedimentation, homogeneous mixtures remain well-mixed and do not settle. This is because the particles within a homogeneous mixture are evenly distributed throughout.
Consistent Taste and Properties
Another way to identify a homogeneous mixture is through its consistent taste and properties. Homogeneous mixtures taste the same throughout, as the composition and proportions of the components are the same in every part of the mixture. Additionally, homogeneous mixtures exhibit consistent properties, such as density, boiling point, and conductivity, throughout the entire volume.
Homogeneous Mixtures Examples
Homogeneous mixtures can be found in various aspects of our daily lives. Here are some examples of homogeneous mixtures:
- Saltwater Solution: When salt (sodium chloride) is dissolved in water, it forms a homogeneous mixture known as saltwater. The salt particles are evenly distributed throughout the water, resulting in a uniform composition.
- Air: Air is considered a homogeneous mixture of gases, primarily nitrogen (about 78%), oxygen (about 21%), and smaller amounts of other gases like argon, carbon dioxide, and trace elements. The components of air are uniformly distributed, creating a consistent composition throughout.
- Sugar Solution: When sugar is dissolved in water, it forms a homogeneous mixture known as a sugar solution. The sugar particles are evenly dispersed in the water, resulting in a uniform sweet taste throughout the mixture.
- Vinegar: Vinegar is a solution of acetic acid in water and is a common example of a homogeneous mixture. The acetic acid molecules are evenly distributed in the water, giving vinegar its characteristic taste and properties.
- Metal Alloys: Alloys are solid homogeneous mixtures of two or more metals. Common examples include brass (a mixture of copper and zinc), bronze (a mixture of copper and tin), and stainless steel (a mixture of iron, chromium, and nickel). Alloys have specific properties that make them useful in various applications, such as strength, corrosion resistance, and conductivity.
- Fruit Juice: Commercial fruit juices are typically homogeneous mixtures of water, natural fruit juices, sweeteners, and sometimes preservatives. The components are thoroughly mixed, resulting in a uniform taste and appearance throughout the juice.
These examples illustrate the wide range of homogeneous mixtures that can be found in our everyday lives. From the fluids we consume to the materials we use, homogeneous mixtures play a crucial role in various industries and applications.
How Kunduz Can Help You Learn Homogeneous Mixtures?
At Kunduz, we are committed to providing you with comprehensive and engaging learning materials to enhance your understanding of chemistry concepts. Our expert educators and resources are designed to simplify complex topics, making learning enjoyable and effective.
Whether you are a student preparing for exams or a curious learner seeking to expand your knowledge, Kunduz can be your reliable companion in your academic journey. With our user-friendly platform, you can access a wide range of courses, videos, practice questions, and interactive materials to suit your learning style and pace.
Join the Kunduz community today and unlock your full potential in chemistry and other subjects. Let us guide you towards academic success and a deeper appreciation for the world of science. Together, we can make learning an empowering and transformative experience.
For readers exploring the concept of homogeneous mixtures and interested in broader chemistry topics, our benzoic acid and Boltzmann constant pages serve as valuable references. These resources offer insights into the chemical properties of benzoic acid and the fundamental role of the Boltzmann constant.
