Inorganic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Inorganic Chemistry
Preparation and Properties of CompoundsWatch the video to determine which of the following relationships are correct according to Boyle's law.
Check all that apply.
V ∝ P
P ∝ 1/V
V ∝ 1/P
PV ∝ V
PV ∝ P
P ∝ V

Inorganic Chemistry
Preparation and Properties of CompoundsWrite the empirical formula of at least four binary ionic compounds that could formed from the following ions:
Ca²+, Cr4+, I-, O²-

Inorganic Chemistry
Qualitative analysisAt 4.00 L, an expandable vessel contains 0.864 mol of oxygen gas. How many liters of oxygen gas must be added at constant temperature and pressure if you need a total of 1.96 mol of oxygen gas in the vessel? Express the volume to three significant figures, and include the appropriate units.

Inorganic Chemistry
Preparation and Properties of CompoundsConsider a 9.02 g sample of C3H6. How many atoms of C would be in this sample?

Inorganic Chemistry
Preparation and Properties of CompoundsA photon having a wavelength in eV. (1 eV = 1.602 x 10^-19 J) of 437 nm strikes a metal surface having a threshold frequency of 3.23 x 10^14 Hz

Inorganic Chemistry
Preparation and Properties of CompoundsAntacids are often used to relieve pain and promote healing in the treatment of mild ulcers. Write balanced net ionic equations for the reactions between the HCl(aq) in the stomach and each of the following substances used in various antacids. MgCO3(s)

Inorganic Chemistry
Qualitative analysisHow many grams of Rb are there in a sample of Rb that contains 1.07×10^24 atoms?

Inorganic Chemistry
Classification of Elements and PeriodicityConsidering periodic trends, valence electrons in which of the following atoms experience the greatest effective nuclear charge (Zeff)?
A) CI
B) C
C) F
D) Ne
E) B

Inorganic Chemistry
Qualitative analysisWhen the following solutions are mixed together, what precipitate (if any) will form?
Note: Leave the answer blank if no precipitate will form.
(Express your answer as a chemical formula.)
KCl(aq) + NaNO3(aq) →
CaCl₂(aq) + Na3PO4(aq) →
NaOH(aq) + Fe2(SO4)3(aq) →

Inorganic Chemistry
Preparation and Properties of CompoundsConsider a 8.12 g sample of C4H10. How many molecules are in this sample?

Inorganic Chemistry
Qualitative analysisConcentrated hydrochloric acid is an aqueous solution that is 35.00 % HCI. The density of the solution is 1.19 g/mL. What mass of HCI is contained in 0.545 L of solution?

Inorganic Chemistry
Preparation and Properties of CompoundsWhen aqueous solutions of lead(II) nitrate and potassium carbonate are combined, solid lead(II) carbonate and a solution of potassium nitrate are formed.

Inorganic Chemistry
Classification of Elements and PeriodicityComplete the table below. For example, in the first row decide whether Sc is a cation or anion. In the second row, write the symbol for the ion that an atom of cesium is mostly likely to form and then decide what type of ion it is.
![The daily output of stomach acid (gastric juice) is 1000 mL to 2000 mL. Prior to a meal, stomach acid (HCl) typically has a pH of 1.42. (10.5, 10.6)
a. What is the [H3O+] of stomach acid?
b. One chewable tablet of the antacid Maalox contains 600. mg of CaCO3. Write the neutralization equation, and calculate the milliliters of stomach acid neutralized by two tablets of Maalox.
c. The antacid milk of magnesia contains 400. mg of Mg(OH)2 per teaspoon. Write the neutralization equation, and calculate the number of milliliters of stomach acid neutralized by one tablespoon of milk of magnesia (one tablespoon = three teaspoons).](https://media.kunduz.com/media/sug-question/raw/75883813-1659281014.3618162.jpeg?w=256)
Inorganic Chemistry
Classification of Elements and PeriodicityThe daily output of stomach acid (gastric juice) is 1000 mL to 2000 mL. Prior to a meal, stomach acid (HCl) typically has a pH of 1.42. (10.5, 10.6)
a. What is the [H3O+] of stomach acid?
b. One chewable tablet of the antacid Maalox contains 600. mg of CaCO3. Write the neutralization equation, and calculate the milliliters of stomach acid neutralized by two tablets of Maalox.
c. The antacid milk of magnesia contains 400. mg of Mg(OH)2 per teaspoon. Write the neutralization equation, and calculate the number of milliliters of stomach acid neutralized by one tablespoon of milk of magnesia (one tablespoon = three teaspoons).

Inorganic Chemistry
Coordination compoundsA 50/50 blend of engine coolant and water (by volume) is usually used in an automobile's engine cooling system. If a car's cooling system holds 4.90 gal, what is the boiling point of the solution?
For the calculation, assume that at normal filling conditions, the densities of engine coolant and water are 1.11 g/mL and 0.998 g/mL respectively. Also, assume that the engine coolant is pure ethylene glycol (HOCH, CH₂OH), which is non-ionizing and non-volatile, and that the pressure remains constant at 1.00 atm. The boiling-point elevation constant for water will also be needed.

Inorganic Chemistry
Classification of Elements and PeriodicityWhich statement best describes the polarity of SCl4F₂?
► View Available Hint(s)
The molecule is always polar.
The molecule is always nonpolar.
Depending on the arrangement of outer atoms, this molecule could be polar or nonpolar.

Inorganic Chemistry
Preparation and Properties of CompoundsCyclohexene reacts with oxygen to produce carbon dioxide and water by the following balanced chemical reaction.
2 C6H10(l) + 17 O2 (g) → 12 CO2 (g) + 10 H₂O (l)
a. If 59.0. grams of O2 is reacted with 24.5 grams of C6H10, which is the limiting reagent? Limiting reagent
b. How many grams of H20 will be produced? Grams
If 10.2 grams of H₂O are produced, what is the % yield for the reaction?
% yield (10 points)

Inorganic Chemistry
Qualitative analysisA solution of 0.263 M HCI is added to a buffer system containing 0.50 M acetic acid and 0.50 M sodium acetate. Find the pH of the solution. The K, of acetic acid is 1.8 x 10¹5. (show answer as a decimal to 3 significant digits) pH =

Inorganic Chemistry
Preparation and Properties of CompoundsDichlorobenzene, C6H4Cl2, exists in three forms (isomers), called ortho, meta, and para:
Which of these has a nonzero dipole moment?
Drag the appropriate items to their respective bins.

Inorganic Chemistry
Classification of Elements and PeriodicityWhich one of the following is the best scientific description of "burning calories"?
Calories are removed from fats leaving carbon dioxide and water behind.
None of these statements is correct.
Fats, proteins or carbohydrates are converted into gases.
Stored nutrients are converted into carbon dioxide and water accompanied by heat release.
Calories in a solid form are converted to calories in a vapor form.
Glycogen combines with carbon dioxide to yield oxygen.

Inorganic Chemistry
Qualitative analysisLDL receptors are
found only in the heart.
None of these responses is correct.
found in the nucleus of cells.
protein molecules on the surface of cells.
easily oxidized.
openings in cell membranes through which cholesterol can pass.

Inorganic Chemistry
Preparation and Properties of CompoundsConsider the acid H3PO4. This acid will react with water by the following equation.
H₂PO4 + H₂<=> H₂PO4 + H₂O
What will be true of the resulting conjugate base H₂PO4?
Select the correct answer below:
H₂Pcan act as an acid.
H₂Pcan act as a base.
H₂PO4 can act as an acid or a base.
depends on the substance

Inorganic Chemistry
Classification of Elements and PeriodicityWhich one of the following concerning the genetically modified purple tomato is correct?
It has been modified to have a higher content of lycopene.
It was created by crossbreeding red tomatoes with plums.
It has been modified to have a higher level of anthocyanins.
It is only on the market in Canada.
It has been modified to produce the Bt toxin.
None of these statements is correct.

Inorganic Chemistry
Preparation and Properties of CompoundsBenzopyrene is a carcinogenic polycyclic aromatic hydrocarbon and
None of these responses is correct.
is an anti-carcinogen.
is a breakdown product of pesticides.
forms when certain foods are heated to a high temperature.
is by far the main pollutant produced by automobiles.
can act as a hormone.

Inorganic Chemistry
Classification of Elements and PeriodicityWhich one of the following statements best describes why champagne is extremely bubbly?
None of these responses is correct.
Fermentation takes place in a closed bottle to retain the CO2 produced.
After fermentation is completed, CO2 is added to a closed bottle.
A secondary fermentation produced from sugar and yeast takes place in a closed bottle.
Fermentation is started by adding CO2 to a closed bottle.
Fermentation takes place in large vats and CO2 gas is then added to the wine.

Inorganic Chemistry
P Block - Group 13Which of the following correctly represents the second ionization of aluminum?
A) Al+ (g) + e-> Al(g)
B) Al+ (g) +e-> AIR+ (g)
C) Al- (g) + e- > A12- (g)
D) Al+ (g) > A12+ (g)+e-
E) AI (g) > Al+ (g) + e-

Inorganic Chemistry
MetallurgyPercival Pott
None of these responses is correct.
was the first to design a randomized double-blind trial for carcinogens in food.
showed that meat cooked at a high temperature was carcinogenic.
described the possibility of cancer being linked to an occupation.
carried out the first cohort study on cancer.
linked smoking to lung cancer.

Inorganic Chemistry
Preparation and Properties of CompoundsUse formal charges to determine the best Lewis structure for ethene, C₂H4. Which of the following statements applies to the structure? Select all that apply.
The structure contains one or more double bonds.
Two or more resonance structures are possible.
The central element has an expanded octet.
One or more atoms does NOT follow the octet rule.
The formal charges of all atoms in the compound are all zero.

Inorganic Chemistry
Qualitative analysisA 7.12 x 10-4 mol sample of KOH is dissolved in water to make up 50.0 mL of solution. What is the pH of the solution at 25.0°C? Round the answer to three significant figures.
Select the correct answer below:
12.2
1.85
15.85
10.9

Inorganic Chemistry
Coordination compoundsGreenhouse gases
Greenhouse gases allow visible light to pass through and warm the Earth's surface, and also allow heat energy to radiate back out into space.
Greenhouse gases prevent visible light from passing through and cooling the Earth's surface, and prevent heat energy from radiating back out into space.
Greenhouse gases allow visible light to pass through and cool the Earth's surface, and prevent heat energy from radiating back out into space.
Greenhouse gases prevent visible light from passing through and warming the Earth's surface, and prevent heat energy from radiating back out into space.
Greenhouse gases allow visible light to pass through and warm the Earth's surface, and prevent heat energy from radiating back out into space.

Inorganic Chemistry
Preparation and Properties of CompoundsWhich of the following statements explains the correct reasoning for the use of molality (rather than molarity) in this equation?
a) As the temperature of a solution changes, its volume will also change, which will affect its molarity but not its molality.
b) In solutions, moles are not directly related to grams and the boiling point of a solution is dependent solely on the number of grams of solute.
c) The equation was originally published with m as a typo, rather than M, but the values are close enough that the equation is still valid.
d) Molality does not appear in many equations, so it is used here to distinguish this equation from other similar ones.

Inorganic Chemistry
Classification of Elements and PeriodicityAfter the final reaction in the copper cycle, the copper solid was rinsed with water and then ethanol. What was being removed from the copper in this process? The equation for the final reaction is given below. Would compounds from previous reactions (not this final reaction) also be removed?
3CuSO4 (aq) + 2Al(s) → 3Cu(s) + Al₂(SO4)3(aq)

Inorganic Chemistry
Qualitative analysisGold is a/an
element
compound
homogeneous mixture
heterogeneous mixture

Inorganic Chemistry
MetallurgyWhat is the GHS classification of the substance or mixture for aluminum? Select all that apply.
Flammable solids
Aquatic toxicity or aquatic toxicity
Specific target organ toxicity - repeated exposure
Respiratory sensitisation
Eye irritation
Not a hazardous substance or mixture
Reproductive toxicity
Skin sensitisation
Oral toxicity
Carcinogenicity

Inorganic Chemistry
Classification of Elements and PeriodicityThe density of osmium (the densest metal) is 22.57 g/cm³. What volume would be occupied by 1.67 kg of osmium?

Inorganic Chemistry
HydrogenWhat kind of intermolecular forces act between a methanol (CH3OH) molecule and a hydrogen fluoride molecule?

Inorganic Chemistry
S Block - Group 1If the temperature of a bowl of ice cream increases from -10°C to 25°C, what is the increase in temperature in units of degrees Celsius and Kelvin?
15°C, 288 K
35°C, 308 K
35°C, 273 K
35°C, 35 K
15°C, 273 K

Inorganic Chemistry
Preparation and Properties of CompoundsThe ability to see light scattering when passed through a colloidal suspension is known as ...
an emulsifying agent effect.
a particle size scattering effect.
the dispersing effect.
the Tyndall effect.

Inorganic Chemistry
D Block elementsAn atom of element number 33 (As) is in its ground electronic state. Which one of the following sets quantum numbers could not apply to any of its electrons?

Inorganic Chemistry
Classification of Elements and PeriodicityAlisha went to the doctor and got her blood calcium levels measured. If her blood calcium is 9.1 mg/dL. How many total grams of calcium are present in her bloodstream? (Alisha has a total blood volume of 3.7 L.)

Inorganic Chemistry
D Block elementsDraw the Lewis structure for CSe2 in the window below and then answer the questions that follow.

Inorganic Chemistry
Qualitative analysisFor the following reaction, 27.5 grams of diphosphorus pentoxide are allowed to react with 14.7 grams of water.
diphosphorus pentoxide (s) + water (l) -> phosphoric acid (aq)
What is the maximum amount of phosphoric acid that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Inorganic Chemistry
Qualitative analysisBalance the following redox reaction in basic solution.
Br (aq) + N₂(g) → Br₂()+N₂H₂(aq)

Inorganic Chemistry
Classification of Elements and PeriodicityDraw the Lewis dot structure for the compounds below. Determine the electronic structure and molecular geometry of those compounds by referring to their Lewis dot structures, the basics sheet provided and your text:
a. oxygen difluoride (OF2):
b. dinitrogen monoxide (N₂O):
c. phosphorus trifluoride (PF3):
d. formaldehyde (CH₂O):
e. carbon tetrafluoride (CF4):

Inorganic Chemistry
Qualitative analysisGaseous methane (CH4) reacts with gaseous oxygen gas (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). What is the theoretical yield of carbon dioxide formed from the reaction of 3.8 g of methane and 9.2 g of oxygen gas?
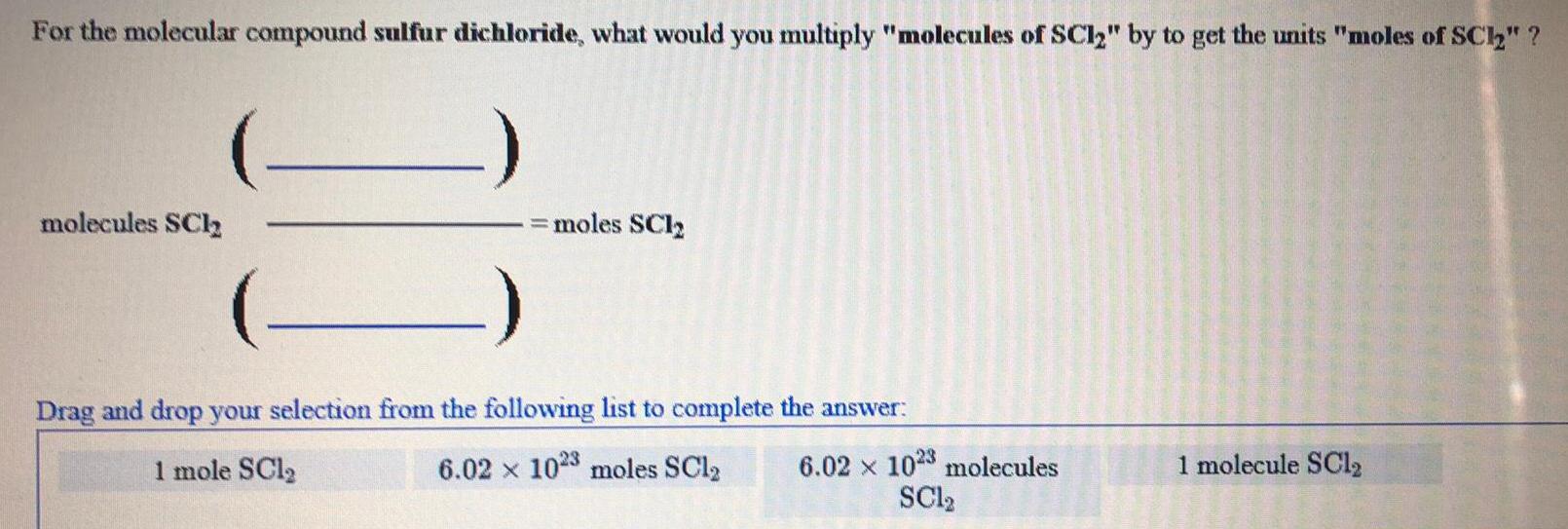
Inorganic Chemistry
Preparation and Properties of CompoundsFor the molecular compound sulfur dichloride, what would you multiply "molecules of SC12" by to get the units "moles of SC1₂" ?

Inorganic Chemistry
Qualitative analysisNitrogen monoxide reacts with oxygen to form nitrogen dioxide
2NO+O₂ -> NO2 ΔH = -114.1 kJ/mole
a) What is the enthalpy change when 1.25 g of NO are converted completely to NO₂?
b) Is this reaction endothermic or exothermic? How do you know?

Inorganic Chemistry
Preparation and Properties of CompoundsThe citric acid in a lemon juice sample was neutralized by titration with a NaOH solution.
H3C6H5O7 + 3 NaOH Na3C6H507 + 3 H₂O
If 5.00 mL of lemon juice required 47.8 mL of 0.121 M NaOH for neutralization, what was the molarity of the citric acid in the lemon juice?

Inorganic Chemistry
Classification of Elements and PeriodicityYour task is to write the chemical formula from each name on your work page. You should show some work to justify the answers but do not need to write a lengthy explanation. You will first match the name to the compound type and then write down the formula on your work page.
>
bartic acid
maggoburnsic acid
dinedogen pentaburnside
homerium(III) nedtite
milhouseum bartine
1. Acid
2. lonic compound
3. Molecular compound

Inorganic Chemistry
Classification of Elements and PeriodicityTotal number of CORRECT orders is :-
(i) PbO₂ > PbO (oxidising strength)
(ii) SiO₂ < NaCl (Melting point)
(iii) Agl > AgCl (Colour intensity)
(iv) ZnCl₂ < HgCl2 (Degree of ionisation in aqueous solution)
(v) Na₂CO3 < MgCO3 (Temperature required for thermal decomposition)