Inorganic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Inorganic Chemistry
Qualitative analysisWhat fraction of the ox particles in Rutherford's gold foil experiment are scattered at large angles? Assume the gold foil is two layers thick, as shown in the figure, and that the approximate diameters of a gold atom and its nucleus are 1.6 Å and 1.2 x 10^-4 A, respectively. Hint: Calculate the cross-sectional area occupied by the nucleus as a fraction of that occupied by the atom. Assume that the gold nuclei in each layer are offset from each other. Express your answer to two significant figures.

Inorganic Chemistry
Qualitative analysisA certain metal M forms a soluble sulfate salt MSO4. Suppose the left half cell of a galvanic cell apparatus is filled with a 2.00 M solution of MSO, and the right half cell with a 10.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C.
Which electrode will be positive?

Inorganic Chemistry
MetallurgyArrange the following elements in order of decreasing ionization energy.
Rank from highest to lowest ionization energy. To rank items as equivalent, overlap the
molybdenum
Reset
silver germanium phosphorus strontium

Inorganic Chemistry
Qualitative analysisWhen 50.0 mL of 1.0 mol/L hydrochloric acid is neutralized completely by 75.0 mL of 1.0 mol/L sodium hydroxide in a coffee-cup calorimeter, the temperature of the total solution changes from 20.2 °C to 25.6 °C. Determine the quantity of energy transferred, q, and state whether the reaction was endothermic or exothermic. [ans: 2800 J; exothermic)

Inorganic Chemistry
P Block - Group 15With respect to periodic properties, the CORRECT statement is
Electron affinity order is F>O > Cl
First ionisation energy order is Al> Mg > K
Atomic radius order is N> P > As
Ionic radius order is K+ > Ca2+ > Mg²+

Inorganic Chemistry
S Block - Group 2Barium sulfate,/BaSO, is a white crystalline solid that is insoluble in water. It is used by doctors to diagnose problems with the digestive system. Banum hydroxide, (Ba(OH),, is also a white crystalline sold and is used in wastewater treatment. How many more oxygen atoms are represented in the formula for barium sulfate than in the formula for barium hydroxide?

Inorganic Chemistry
MetallurgyWhy do stars seem to twinkle?
Shifting currents of air low in the atmosphere bend starlight, causing it to miss our eyes in fractions of moments.
Stars are so far out in space that other orbiting objects in space block our view at certain moments.
Starlight is so bright that our eyes protect us by filtering out some of the light.
Starlight is light from dying stars that give off interrupted light.

Inorganic Chemistry
Preparation and Properties of CompoundsA perfect cube of Al metal was found to have a mass of 16.2 g. If the density of Al is 2.7 g/cm³, then the length of each side of the aluminum cube is

Inorganic Chemistry
Metallurgy(a) Read the following description of the element zinc and indicate which are physical properties and which are chemi- cal properties. Zinc melts at 420 °C. When zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. Zinc has a hardness on the Mohs scale of 2.5 and a density of 7.13 g/cm³ at 25 °C. It reacts slowly with oxygen gas at ele- vated temperatures to form zinc oxide, ZnO.
(b) Which properties of zinc can you describe from the photo? Are these physical or chemical properties?

Inorganic Chemistry
Preparation and Properties of CompoundsAmino acids make peptides and proteins. Draw the structures of the following compound between parentheses: The amino acid (Alanine), (Tryptophan), (Tyrosine) and (a- amino, ß-hydroxy butanoic acid)

Inorganic Chemistry
Preparation and Properties of CompoundsRound the following numbers to 4 significant figures and write the rounded number on the line. Use scientific notation if needed to get the correct number of significant figures. (1 point per answer, 4 points total)
a. 0.00470060
b. 439990
c. 1.2456x10^4
d. 2.0501

Inorganic Chemistry
Qualitative analysisA 2.57 L sample of O₂ has a measured pressure of 920 mmHg and temperature of 30°C. a) How many moles of gas are present in the sample? b) Would you expect the answer to be higher, lower or the same for H₂ gas at same conditions in the same 2.57 L container? Explain.

Inorganic Chemistry
Classification of Elements and PeriodicityWhen copper is bombarded with high-energy electrons, X-rays are emitted. Calculate the energy, in joules, associated with the photons if the wavelength of the x-rays is 0.154 nm.

Inorganic Chemistry
Qualitative analysisHow many types of orbitals are there in the shell with n=3 in an atom?

Inorganic Chemistry
Classification of Elements and PeriodicityWhat did Rutherford expect to happen when he aimed particles at the gold foil?
Rutherford expected the particles to stop moving after touching the gold foil.
Rutherford expected the particles to reflect from the gold foil and return in the opposite direction.
Rutherford expected the particles to react with gold atoms forming a new compound
Rutherford expected the particles to travel in straight paths through the gold foil.
Rutherford expected the particles to be deflected from their straight paths as they passed through the gold foil.

Inorganic Chemistry
Preparation and Properties of CompoundsDrago's rule is used, when we compare compounds having different central atom but same surrounding atoms. It says lone pair occupies stereo chemically inactive "S" orbital Which of the following do not obey drago rule.
CH₁
ASH
H₂Se
BiH,

Inorganic Chemistry
MetallurgyOne sunflower pot is watered with pure water and 3 others are watered with increasing concentrations of salt water. The height of the sunflowers is measured weekly. What is the independent variable in this investigation?
the pure water
the concentration of salt water
height of the sunflowers
the species of flower

Inorganic Chemistry
Qualitative analysisCalculate the heat change in kilojoules for condensation of 175 g of steam at 100 °C. Express your answer as a positive value using three significant figures and include the appropriate units.
Inorganic Chemistry
Preparation and Properties of CompoundsWhat is the specific heat (J/g °C) of the metal? Express your answer using two significant figures.

Inorganic Chemistry
Preparation and Properties of CompoundsCaroline takes a bath containing 8.5 kg of water, but as time passes it cools off to 19°C. James doesn't want to follow his sister in a cold bath, so he asks for more water to be added. How much water do I need to add to the tub if the water is 49°C when it comes out of the faucet so that the final temperature in the tub is 38°C? (Put your answer in kilograms - no units in Canvas)

Inorganic Chemistry
S Block - Group 2Calcium and chlorine form CaCl₂ rather than CaCl because
less energy is required to remove one electron from the calcium atom than to remove two electrons
more energy is released in forming chloride ions from chlorine molecules in the formation of CaCL(s)
the lattice energy of CaCl (s) is less exothermic than of CaCl₂ (s)
CaCl (s) is formed its elements, more energy is released than when CaCl₂ (s) is formed from its elements

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the heat change in kilocalories for condensation of 9.0 kg of steam at 100 °C. Express your answer as a positive value using two significant figures and include the appropriate units.

Inorganic Chemistry
Classification of Elements and PeriodicityGiven that an atom of a semiconductor has a diameter of 2.40 Å, what is the maximum number of moles that fit in the channel of a processor chip that is 67.4 nm long, 85.0 nm, wide and 285 nm thick? Assume that the atoms stack in a simple cubic arrangement. Express your answer numerically in moles.
![Condiser the following solutions or compounds :
(i) [He(g) + O₂(g)]
(ii) HCI in aq. solution
(iii) NaCl in aq. solution
(iv) NH3 (9)
Total number of cases where ion-dipole interaction is present = x
Total number of cases where hydrogen bonding is present = y
Total number of cases where only London dispersion forces are present = z
The value of √(y+z) / x is](https://media.kunduz.com/media/sug-question/raw/53685255-1657494063.5734103.jpeg?w=256)
Inorganic Chemistry
Preparation and Properties of CompoundsCondiser the following solutions or compounds :
(i) [He(g) + O₂(g)]
(ii) HCI in aq. solution
(iii) NaCl in aq. solution
(iv) NH3 (9)
Total number of cases where ion-dipole interaction is present = x
Total number of cases where hydrogen bonding is present = y
Total number of cases where only London dispersion forces are present = z
The value of √(y+z) / x is

Inorganic Chemistry
Qualitative analysisAn acetaminophen suspension for infants contains 80 mg/ 0.80 mL suspension. The recommended dose is 15 mg/kg body weight. How many mL of this suspension should be given to an infant weighing 14 lb? (Assume two significant figures.)

Inorganic Chemistry
MetallurgySelect all that apply
Which of the following options correctly describe the mass number of an element? Select all that apply.
The mass number is given by the sum of the protons and neutrons in the nucleus.
The mass number is often written as a left superscript next to the atomic symbol.
The mass number of an element is given the symbol A.
The mass number of a particular element never varies.
In a neutral atom, the mass number equals the number of electrons in the atom.

Inorganic Chemistry
Classification of Elements and PeriodicityFrom this description, identify the physical and chemical properties and if they are intensive or extensive: Zinc melts at 420°C. When zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. Zinc has a hardness on the Mohs scale of 2.5 and a density of 7.13 g/cm² at 25°C. It reacts slowly with oxygen gas at elevated temperatures to form zinc oxide, ZnO.

Inorganic Chemistry
MetallurgyWhich of the following options correctly describe the mass number of an element? Select all that apply.
The mass number is given by the sum of the protons and neutrons in the nucleus.
The mass number is often written as a left superscript next to the atomic symbol.
The mass number of an element is given the symbol A.
The mass number of a particular element never varies.
In a neutral atom, the mass number equals the number of electrons in the atom.

Inorganic Chemistry
Classification of Elements and PeriodicityLabel the particle diagrams below with the letter of one of the following categories.
(Categories may be used once, more than once, or not at all):
a. Element
b. Compound
c.Mixture of elements
d.Mixture of elements and compounds
e. Mixture of compounds

Inorganic Chemistry
Qualitative analysisAssertion (A): Every state is sovereign; it has supreme and absolute power within its territory and can decide its foreign and domestic policies.
Reason (R): For ages, the idea of a state's sovereignty has been closely associated with the concept of the state's ability to ensure the welfare and well-being of its citizens.
Which of the following is TRUE for the above statements?
Both (A) and (R) are true, but (R) is not the correct explanation of A.
Both (A) and (R) are true, and (R) is the correct explanation of A.
A is false, and (R) is true.

Inorganic Chemistry
Classification of Elements and PeriodicityThe thermite reaction is used to weld massive metal parts such as the propellers for large ships. How many kilograms of iron(III) oxide (159.7 g/mol) are needed to generate 1.20 x 10^6 kJ of heat?
Fe₂O3(s) + Al(s)→ Al₂O3(s) + 2 Fe(s) ΔH = -848 kJ/mol
![The equilibrium reaction below has the following equilibrium mixture concentrations: SO3 = [0.0894],
SO₂ = [0.400] and O2 = [0.200] with Kc = 4.00
If the concentration of O2 at equilibrium is decreased to [0.100], how and for what reason will the equilibrium shift. Be sure to calculate the value of the reaction quotient, Q, and use this to confirm your answer.
2 SO3 (g) ↔ 2 SO2 (g) + O2(g)](https://media.kunduz.com/media/sug-question/raw/53278552-1657493013.553849.jpeg?w=256)
Inorganic Chemistry
Preparation and Properties of CompoundsThe equilibrium reaction below has the following equilibrium mixture concentrations: SO3 = [0.0894],
SO₂ = [0.400] and O2 = [0.200] with Kc = 4.00
If the concentration of O2 at equilibrium is decreased to [0.100], how and for what reason will the equilibrium shift. Be sure to calculate the value of the reaction quotient, Q, and use this to confirm your answer.
2 SO3 (g) ↔ 2 SO2 (g) + O2(g)

Inorganic Chemistry
Preparation and Properties of CompoundsTo help us get better at naming molecules that contain two or more functional groups, I am going to have you submit a picture of a molecule that has two functional groups, with at least one of the functional groups being a C=O group.
Here are a few other rules for your structure:
It must be different than the molecules in your notes and problem set.
It must be different from any of your classmates posted below (I've allowed you to see other answers before you post.) This isn't as hard as it sounds, change from a 5 membered ring to a 7 membered ring, change from carboxylic acid to ester, change from cyclic parent chain to acyclic, etc. Make sure you are able to name that molecule based on the information in the text. If I am unable to name the molecule you've provided, I will deduct 5 points from your grade, per structure. Make sure the image is embedded in the thread and not attached as something you must download to see.
You do not have to explain anything when you post your image, just post them.

Inorganic Chemistry
Qualitative analysisThe process of causing matter to move against an opposing force is
potential energy
kinetic energy
thermal energy
work
Inorganic Chemistry
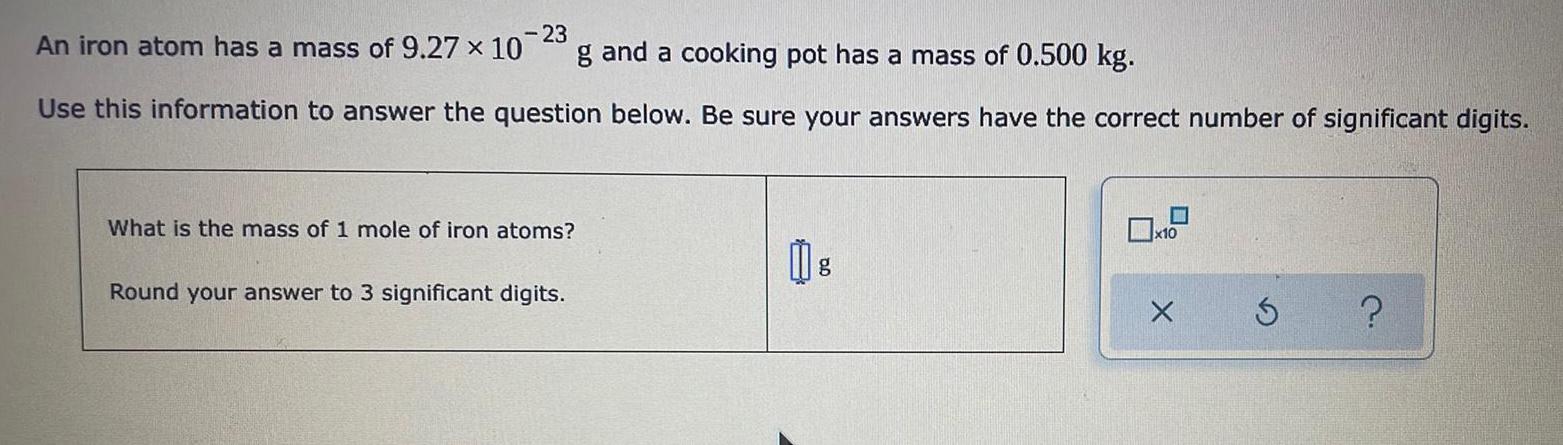
S Block - Group 1An iron atom has a mass of 9.27 x 10^-23 g and a cooking pot has a mass of 0.500 kg.
Use this information to answer the question below. Be sure your answers have the correct number of significant digits.

Inorganic Chemistry
Preparation and Properties of CompoundsA chemist determines by measurements that 0.0450 moles of chlorine gas participate in a chemical reaction. Calculate the mass of chlorine gas that participates. Round your answer to 3 significant digits.
![For the coordination compound [Ni(H₂O)4]I2, the number of unpaired electrons and magnetism would be
0 and paramagnetic
0 and diamagnetic
2 and diamagnetic
4 and paramagnetic
4 and diamagnetic
2 and paramagnetic](https://media.kunduz.com/media/sug-question/raw/72676265-1657492811.5918076.jpeg?w=256)
Inorganic Chemistry
Coordination compoundsFor the coordination compound [Ni(H₂O)4]I2, the number of unpaired electrons and magnetism would be
0 and paramagnetic
0 and diamagnetic
2 and diamagnetic
4 and paramagnetic
4 and diamagnetic
2 and paramagnetic

Inorganic Chemistry
Classification of Elements and PeriodicityWork is
the capacity to supply heat or do work
the process of causing matter to move against an opposing force
the energy an object possesses because of its relative position, composition, or condition
the energy that an object possesses because of its motion

Inorganic Chemistry
Preparation and Properties of CompoundsFor the Lewis electron dot formula for hydrogen silicon trichloride, HSICI3, there are REDS around the Si and the shape is
note: Add a number, then a letter.
a) planar trigonal
b) linear
c) diatomic
d) tetrahedral
e) pyramidal
f) bent

Inorganic Chemistry
Classification of Elements and PeriodicityXeCl4 has
an octahedral electron pair geometry, and a square planar molecular shape
a square planar electron pair geometry, and an octahedral molecular shape
a trigonal bipyramid electron pair geometry, and a seesaw molecular shape
a tetrahedral electron pair geometry, and a tetrahedral molecular shape

Inorganic Chemistry
Classification of Elements and PeriodicityA cyclotron is a device that can accelerate charged particles to very high velocities, and can be used to generate protons with radiative wavelengths that can kill cancer cells. What is the de Broglie wavelength of a proton (mass 1.673 x 10^-27 kg) that has been accelerated to a velocity of 1.35 x 10^4 m/s?
2.93 x 10^-11 m
5.35 x 10^-3 m
3.41 x 10^10 m
1.67 x 10^-10 m

Inorganic Chemistry
Preparation and Properties of CompoundsHow many molecules of oxygen, O2, would 7.80 g of Al(OH)3 produce?
1.42 x 10^22 molecules of O₂
4.09 x 10^22 molecules of O₂
9.02 x 10^22 molecules of O₂
5.06 x 10^22 molecules of O₂
8.42 x 10^22 molecules of O₂

Inorganic Chemistry
Qualitative analysisHow many μm are in 18.4 mm?
1.84 x 10^4 μm.
1.84 x 10¹ μm
1.84 x 10^-4 μm
1.84 x 10^-5 μm

Inorganic Chemistry
HydrogenA sample of polystyrene, which has a specific heat capacity of 1.880 J.g¹.C-¹, is put into a calorimeter (see sketch at right) tha contains 100.0 g of water. The polystyrene sample starts off at 91.5 °C and the temperature of the water starts off at 25.0 °C. When the temperature of the water stops changing it's 37.8 °C. The pressure remains constant at 1 atm. Calculate the mass of the polystyrene sample. Be sure your answer is rounded to 3 significant digits.

Inorganic Chemistry
Preparation and Properties of CompoundsA piece of solid gold (specific heat 0.129 J/g °C) at 227.4 °C is placed into 50.0 mL of water at 24.1 °C. The final temperature of both the metal and water is 26.2 °C. Assuming that all heat is transferred between the metal and water, what is the mass of the piece of metal?
16.9 g
172 g
0.439 g
12.4 g

Inorganic Chemistry
HydrogenGiven the following information, what is the standard enthalpy change for the reaction?
O2(g) + 2SO2(g) + → 2SO3(g)
ΔHf SO2(g) = -296.83 kJ/mol
ΔHf O2(g) = 0 kJ/mol
ΔHf SO3(g) = -395.72 kJ/mol
-197.78 kJ
197.78 kJ
-98.89 kJ
98.89 kJ

Inorganic Chemistry
Preparation and Properties of Compounds4. (8 pts) A sample of an amino acid decomposes into 5.851 grams of carbon, 1.145 grams of hydrogen, 2.274 grams of nitrogen, and 2.598 grams of oxygen.
a. (2pts) What is the mass percent of each element in this compound?
C: 49.30%
H: 9.648%
N: 19.16%
O: 21.89%)
b. (1pt) What was the mass of the sample decomposed?
11.868 grams
c. (2pts) What is the empirical formula of the compound?
(C2H7NO)
d. (1pt) What is the empirical formula mass of the compound?
73.1 g/mol
e. (1pt) If the molar mass is 146.2 g/mol, what is the molecular formula?
(C6H14N2O2)
f. (1pt) How many moles of the sample were decomposed?
(0.0812 mol)

Inorganic Chemistry
Classification of Elements and PeriodicityGreenland Ice Sheet Precipitation (GISP) is a common reference material when making hydrogen isotope measurements. Analysis of GISP shows that it is 0.012624% 2H (mass 2.01410 amu) and the remainder is ¹H (mass 1.00782 amu). What is the average mass of GISP?
1.00795 amu
2.02998 amu
1.00783 amu
1.00789 amu

Inorganic Chemistry
Classification of Elements and PeriodicityWhich of the following ions have noble gas electron configurations? Check all that apply.
Mg+
Sc³+
Ga3+
p3-

Inorganic Chemistry
Classification of Elements and PeriodicityDetermining Molecular Formulas from Percent Composition
Steps 1-3
Assume you have a 100-g sample and determine the amount of each element in 100 g of the compound.
Steps 4-5
Find the ratios of amounts of each element to the smallest amount.
Step 6
Use the ratios to determine the empirical formula.
Step 7
Determine the molar mass of the empirical formula.
Step 8
Determine the ratio of the molar masses of the compound and of the empirical formula. This ratio is the number of empirical formula units in the molecule.
Step 9
Multiply the number of atoms of each element in the empirical formula units by the ratio found in the preceding step.
An organic acid is composed of carbon (68.84%), hydrogen (4.96%), and oxygen (26.20%). Its molar mass is 122.12 g/mol. Determine the molecular formula of the compound.