Carbon dioxide (CO2) is a compound that plays a crucial role in various fields including chemistry, biology, and environmental science. Understanding the structure and properties of CO2 is essential for comprehending its behavior and its impact on the environment. In this article, we will explore the Lewis structure, molecular geometry, polarity, and hybridization of CO2, providing a comprehensive understanding of this fascinating molecule.
An Introduction to CO2 Lewis Structure
Lewis structures, also known as electron dot diagrams, were introduced by Gilbert N. Lewis in 1916. They are a visual representation of the arrangement of valence electrons among atoms in a molecule. Lewis structures enable us to predict the physical and chemical properties of molecules by understanding the distribution of electrons.
The Lewis structure of CO2 is particularly interesting because it showcases the bonding and interaction between carbon and oxygen atoms. It demonstrates the concept of valence electrons and how they determine the formation of chemical bonds.
What is Carbon Dioxide?
Before diving into the Lewis structure of CO2, let’s briefly understand what carbon dioxide is. Carbon dioxide is a colorless and odorless gas composed of one carbon atom bonded to two oxygen atoms. It is a naturally occurring compound that is produced through various natural and human activities, including respiration, combustion, and industrial processes.
CO2 is a greenhouse gas, meaning it has the ability to trap heat in the atmosphere. It plays a significant role in regulating Earth’s temperature and climate. However, excessive CO2 emissions from human activities, such as burning fossil fuels, contribute to the greenhouse effect and global warming.

Lewis Structure of Carbon Dioxide
The Lewis structure of CO2 begins with the central carbon atom, which forms double bonds with two oxygen atoms on either side. To determine the Lewis structure of CO2, we need to consider the valence electrons of carbon and oxygen atoms.
- Valence electrons: Carbon has four valence electrons, while each oxygen atom has six. The total number of valence electrons in a CO2 molecule is, therefore, 4 + 2(6) = 16.
- Formation of bonds: Carbon forms double bonds with each oxygen atom, with each bond consisting of two shared electron pairs.
- Resulting structure: The resulting Lewis structure of CO2 shows a carbon atom at the center with double bonds to two oxygen atoms. Each oxygen atom also has two lone pairs of electrons.
The Lewis structure provides a visual representation of the electron distribution in CO2, highlighting the importance of valence electrons in determining how atoms interact and form bonds.
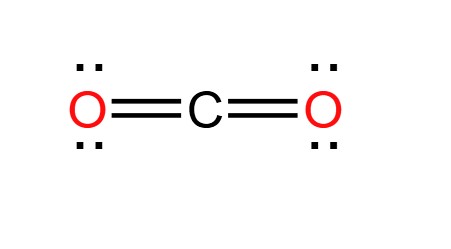
The Basics of Lewis Structures
Before delving deeper into the Lewis structure of CO2, it is essential to understand the basics of Lewis structures. Lewis structures are shorthand notations used by scientists to describe the distribution of electrons in molecules. They help visualize how atoms share or transfer electrons to form bonds and how lone pairs of electrons can affect a molecule’s behavior.
Here are the key elements represented in Lewis structures:
- Atoms: Each atom in a molecule is represented by its chemical symbol.
- Bonds: The bonds between atoms are depicted as lines. A single line represents a single bond (two electrons), a double line represents a double bond (four electrons), and a triple line represents a triple bond (six electrons).
- Lone pairs: Lone pairs, or non-bonding pairs of electrons, are shown as dots. They can affect a molecule’s shape and reactivity.
By learning to read and interpret Lewis structures, you can gain a deeper understanding of the bonds that hold atoms together and the electron distribution within molecules.
How to Draw Lewis Structure of CO2?
Drawing the Lewis structure of CO2 involves several steps to ensure accuracy and capture the electron distribution correctly. Let’s break down the process into simple steps:
- Determine Total Number of Electrons of the Valence Shells of Carbon and Oxygen Atoms:
- Carbon has four valence electrons, while each oxygen atom has six valence electrons. The total number of valence electrons in CO2 is 4 + 2(6) = 16.
- Total Electrons Pairs Existing as Lone Pairs and Bonds:
- Each bond consists of two shared electrons, and each lone pair consists of two unshared electrons. Count the total number of electron pairs, including bonds and lone pairs. In CO2, there are a total of 8 electron pairs.
- Determine Center Atom and Drawing the Sketch:
- The central atom in CO2 is carbon. Carbon is less electronegative than oxygen, making it the most likely candidate for the central atom. Draw a sketch of CO2, placing the carbon atom in the center.
- Mark Lone Pairs on Atoms:
- Start marking lone pairs on the atoms, beginning with the oxygen atoms. Each oxygen atom in CO2 will have three lone pairs of electrons around it. Mark the lone pairs on the oxygen atoms in your sketch.
- Mark Charges on Atoms:
- Check if there are any charges on the atoms. In the case of CO2, there are no charges on the carbon or oxygen atoms.
- Check the Stability and Minimize Charges on Atoms by Converting Lone Pairs to Bonds to Obtain the Best Lewis Structure:
- Analyze the stability of the structure and minimize charges on the atoms. If possible, convert lone pairs to bonds to achieve the best Lewis structure. In the case of CO2, there are no charges, and the structure is already stable.
By following these step-by-step instructions, you can accurately draw the Lewis structure of CO2 and understand the electron distribution within the molecule.
Molecular Geometry of CO2
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. It provides insights into the shape of the molecule, which is crucial for determining its polarity, reactivity, phase of matter, color, magnetism, and biological activity.
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of individual molecules based on the number of electron pairs surrounding the central atom. According to the VSEPR theory, electron pairs arrange themselves to minimize repulsion, resulting in specific geometric shapes for molecules.
In the case of CO2, the carbon atom has two electron groups, both shared with the oxygen atoms through double bonds. There are no lone pairs of electrons around the central carbon atom. As a result, the two electron groups arrange themselves in a straight line, leading to a linear molecular geometry for CO2. The bond angle in CO2 is 180°.
Understanding the molecular geometry of CO2 is essential for predicting its physical and chemical properties, as well as its behavior in various reactions.

Hybridization in CO2
Hybridization is a theoretical concept in chemistry that describes the rearrangement of atomic orbitals in atoms with multiple bonds. This process results in hybrid orbitals, which have different shapes and energies than the original atomic orbitals.
Hybridization can be visualized as a blending of atomic orbitals within an atom. It allows for the explanation of molecule shapes that cannot be described by the valence bond theory alone. There are several types of hybridization, including sp, sp2, and sp3.
In the case of CO2, the carbon atom undergoes sp hybridization. This occurs because the carbon atom has two regions of electron density from the two double bonds with oxygen. The carbon atom uses one s and one p orbital to form two sp hybrid orbitals, which are used to form sigma bonds with the oxygen atoms. The oxygen atoms retain their unhybridized p orbitals, which overlap with the carbon’s sp hybrid orbitals to form π bonds. This results in a molecule with a linear shape.
Hybridization in CO2 significantly influences its molecular geometry and chemical reactivity. Understanding the hybridization of CO2 helps us comprehend its unique properties and behavior in various chemical reactions.
Polarity of CO2
Electronegativity plays a crucial role in determining the polarity of molecules. Electronegativity is a measure of how strongly an atom attracts electrons in a bond. It helps us determine the type of bond formed between atoms and the overall polarity of a molecule.
In the case of CO2, both the carbon and oxygen atoms share electrons, indicating that the bonds are covalent. To determine the polarity of CO2, we need to consider both electronegativity and molecular geometry.
Carbon has an electronegativity value of 2.55, while oxygen has a value of 3.44. The difference in electronegativity values between carbon and oxygen is 3.44 – 2.55 = 0.89, which is less than 1.7. This indicates a polar covalent bond between carbon and oxygen.
However, due to the linear geometry of the CO2 molecule, the dipole moments of the two C=O bonds cancel each other out, making the overall molecule nonpolar. Although the individual bonds in CO2 are polar, the symmetric distribution of the atoms and the cancellation of dipole moments result in a nonpolar molecule.
Understanding the polarity of CO2 is crucial for predicting its solubility, boiling point, and reactivity with other molecules.
CO2 and Electronegativity
Electronegativity is a fundamental concept in chemistry that helps us understand the bonding behavior of atoms. It determines how strongly an atom attracts electrons in a chemical bond.
In the case of CO2, carbon has an electronegativity value of 2.55, while oxygen has a value of 3.44. The difference in electronegativity values between carbon and oxygen is 0.89, which is less than 1.7. This indicates a polar covalent bond between carbon and oxygen.
The electronegativity difference between atoms in a molecule is essential in determining the type of bond formed. In CO2, the polar covalent bonds between carbon and oxygen contribute to the overall polarity of the molecule.
Understanding the role of electronegativity in CO2 is crucial for predicting its bonding behavior and the type of interactions it has with other molecules.
CO2 Bond Angle
The bond angle in a molecule refers to the angle formed by two bonds originating from the same atom. In the case of CO2, the carbon atom is bonded to two oxygen atoms, resulting in a linear molecular geometry.
The linear geometry of CO2 leads to a bond angle of 180°. This means that the two oxygen atoms are positioned in a straight line on opposite sides of the carbon atom.
The bond angle in CO2 is determined by the repulsion between the electron pairs around the central carbon atom. The two double bonds between carbon and oxygen atoms cause the oxygen atoms to arrange themselves as far apart as possible, resulting in a linear shape and a bond angle of 180°.
Understanding the bond angle in CO2 is crucial for predicting its molecular shape and the arrangement of atoms within the molecule.
Resonance Structure of CO2
Resonance structures are alternative Lewis structures that can be drawn for a molecule when there are multiple ways to distribute the electrons. Resonance occurs when there is more than one valid Lewis structure for a molecule.
In the case of CO2, there is only one valid Lewis structure due to the linear geometry and the equal distribution of the double bonds between the carbon and oxygen atoms. Therefore, CO2 does not exhibit resonance.
Resonance structures are particularly important when explaining the stability and reactivity of molecules. In the case of CO2, the absence of resonance structures simplifies the understanding of its properties.

Geometrical Structure of Carbon Dioxide (CO2)
The geometrical structure of carbon dioxide (CO2) refers to the arrangement of atoms in three-dimensional space. While the molecular geometry of CO2 is linear, the geometrical structure takes into account the positions of the atoms in relation to each other.
In CO2, the carbon atom is at the center, with two oxygen atoms on either side. The linear molecular geometry means that the carbon and oxygen atoms are arranged in a straight line, with a bond angle of 180°.
The geometrical structure of CO2 is important for understanding its physical properties, such as its shape, size, and interactions with other molecules. The linear arrangement of atoms in CO2 influences its behavior and reactivity in chemical reactions.
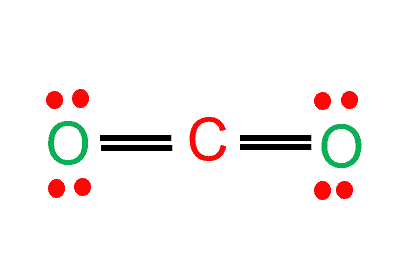
Solved Examples on Lewis Structure of Carbon Dioxide
Let’s solve a few examples to solidify our understanding of the Lewis structure of carbon dioxide (CO2):
Example 1: Draw the Lewis structure of CO2.
Solution:
- Determine the total number of valence electrons:
- Carbon has 4 valence electrons, and each oxygen atom has 6 valence electrons. Therefore, the total number of valence electrons is 4 + 2(6) = 16.
- Determine the total electron pairs:
- The total electron pairs are determined by dividing the total valence electrons by 2. In this case, there are 16/2 = 8 electron pairs.
- Determine the center atom and draw the sketch:
- Since carbon is less electronegative than oxygen, it will be the central atom. Draw a sketch of CO2 with carbon in the center and the oxygen atoms on either side.
- Mark lone pairs on atoms:
- Mark three lone pairs on each oxygen atom, as oxygen can accommodate up to 8 valence electrons.
- Mark charges on atoms:
- In the case of CO2, there are no charges on the carbon or oxygen atoms.
- Check stability and minimize charges:
- The Lewis structure of CO2 is already stable, with no charges on the atoms.
Example 2: Determine the molecular geometry and bond angle of CO2.
Solution: Since CO2 has a linear molecular geometry, the bond angle between the carbon-oxygen bonds is 180°. The linear shape is a result of the repulsion between the electron pairs around the central carbon atom.
By solving these examples, we can practice and reinforce our understanding of the Lewis structure, molecular geometry, and bond angle of carbon dioxide.
How Kunduz Can Help You Learn Lewis Structure of Carbon Dioxide?
At Kunduz, we are dedicated to providing comprehensive and accessible learning resources for students. If you are looking to enhance your understanding of the Lewis structure of carbon dioxide and related concepts, our platform offers a wide range of educational materials.
Our expert tutors will guide you step-by-step through the process of drawing the Lewis structure of CO2 and understanding its molecular geometry, polarity, and hybridization. With Kunduz, you can master the Lewis structure of carbon dioxide and unlock your potential in chemistry. Join us today and embark on an exciting journey of learning and academic excellence.
Related Topics:
