Ozone, also known as O3, is a vital molecular compound that plays a significant role in our atmosphere. It is a pale blue gas composed of three oxygen atoms bonded together. Understanding the Lewis structure of ozone is essential for comprehending its chemical properties, such as its molecular geometry, hybridization, and polarity. In this article, we will explore the intricacies of ozone, its Lewis structure, and various related concepts.
An Introduction to O3 (Ozone) Lewis Structure
Before we delve into the Lewis structure of ozone, let’s first understand the basics of oxygen. Oxygen is a chemical element present in the periodic table with the symbol O and atomic number 8. It belongs to group VI A, which means it has six valence electrons. Ozone, on the other hand, is a molecule consisting of three oxygen atoms bonded together.
Lewis Structure of Ozone (O3)
The Lewis structure of a molecule represents the arrangement of its atoms and valence electrons. It provides valuable insights into the bonding and electronic configuration of the molecule. To determine the Lewis structure of ozone, we need to follow a step-by-step approach.

Step 1: Calculate the Total Number of Valence Electrons
The first step in drawing the Lewis structure of any molecule is to determine the total number of valence electrons. Valence electrons are the electrons present in the outermost energy level of an atom and are involved in chemical bonding.
In ozone, each oxygen atom contributes six valence electrons. Since there are three oxygen atoms in ozone, the total number of valence electrons is 3 * 6 = 18.
Step 2: Select the Central Atom
To identify the central atom in a molecule, we typically look for the least electronegative atom or the atom with the lowest ionization energy. In the case of ozone, all three oxygen atoms are identical, so we can choose any one of them as the central atom.
Step 3: Put Two Electrons Between the Atoms to Represent a Chemical Bond
Next, we connect the outer atoms to the central atom by drawing single bonds between them. Each single bond corresponds to a pair of electrons. In the Lewis structure of ozone, we have one double bond and one single bond between the oxygen atoms.
Step 4: Complete the Octet (or Duplet) on the Outside Atom
In this step, we focus on completing the octet (or duplet in the case of hydrogen) on the outer atoms. An octet refers to having eight valence electrons around an atom, which makes it stable.
For each oxygen atom, we need to add electrons until the octet is complete. Since oxygen has six valence electrons, we add two more electrons to each oxygen atom in the form of lone pairs.
Step 5: Check Whether the Central Atom Has Octet or Not
After completing the octets of the outer atoms, we check whether the central atom has an octet or not. If the central atom does not have an octet, we can move electron pairs from the outer atoms to form double or triple bonds.
In the case of ozone, the central oxygen atom has only six valence electrons. To satisfy the octet rule, we can move one electron pair from one of the outer oxygen atoms to form a double bond with the central oxygen atom. This results in a resonance structure, where the double bond shifts between the outer oxygen atoms.
Step 6: Check the Stability of the Lewis Structure
After completing the Lewis structure, we need to check the stability of the molecule by calculating the formal charge on each atom. The formal charge is a measure of the distribution of electrons in a molecule and helps us determine the most stable arrangement.
To calculate the formal charge, we use the formula: Formal Charge = Valence Electrons – (Number of Lone Pair Electrons + 1/2 * Number of Bonded Electrons)
By following these steps, we can obtain the Lewis structure of ozone.
Molecular Geometry of Ozone (O3)
The molecular geometry of a molecule is determined by the arrangement of its atoms in space. For ozone, the molecular geometry is “bent” or “angular,” which means that the molecule is not linear. This bent shape is a result of the repulsion between the lone pairs of electrons and the bonded electron pairs.
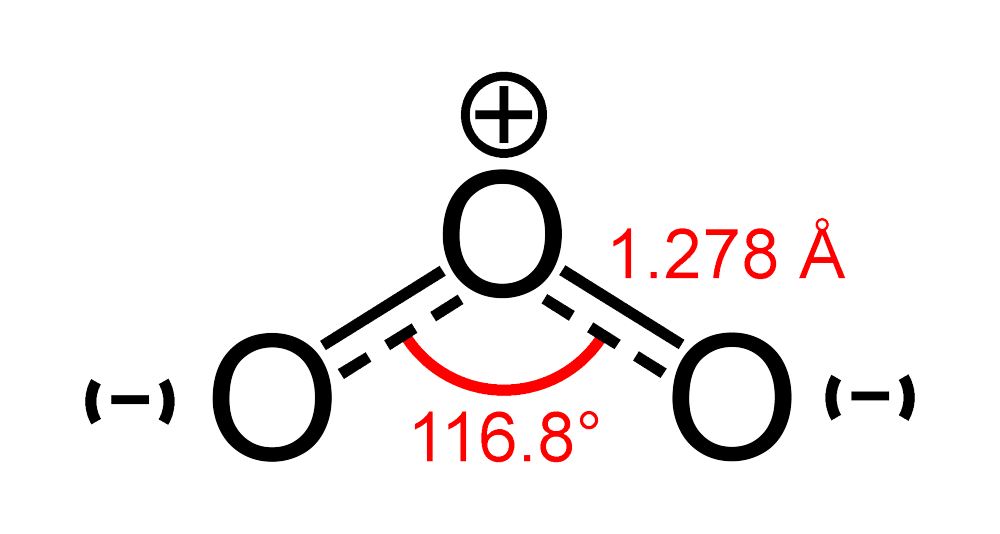
The bond angle in ozone (O3) is approximately 116.8 degrees, which is slightly less than the ideal angle of 120 degrees expected for a trigonal planar geometry. This deviation is due to the repulsion between the lone pairs of electrons, which pushes the bonded oxygen atoms closer together.
Molecular Orbital Diagram of Ozone (O3)
To understand the electronic structure of ozone, we can use a molecular orbital diagram. Molecular orbitals are formed by the combination of atomic orbitals from different atoms. In ozone, the molecular orbital diagram shows the distribution of electrons in the bonding and antibonding orbitals.
The molecular orbital diagram of ozone reveals that there are two pi bonds and one sigma bond. The pi bonds result from the side-to-side overlap of the 2p orbitals of the oxygen atoms, while the sigma bond is formed from the head-on overlap of the 2s orbitals.
Hybridization in O3
Hybridization is a concept used to describe the mixing of different atomic orbitals to form new hybrid orbitals. In ozone, the central oxygen atom undergoes sp2 hybridization, while the outer oxygen atoms maintain their original 2p orbitals.
The sp2 hybridization of the central oxygen atom occurs when one 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar geometry around the central oxygen atom.
The hybridization of the outer oxygen atoms remains unchanged, and they retain their original 2p orbitals.
Polarity and Dipole Moment of Ozone (O3)
Polarity in a molecule refers to the presence of an uneven distribution of electron density, resulting in regions of partial positive and partial negative charges. The dipole moment is a measure of the polarity of a molecule.
In the case of ozone, the molecule is polar due to the presence of a bent molecular geometry. The oxygen atoms have different electronegativities, resulting in an uneven distribution of electron density. This creates a dipole moment in which one end of the molecule is slightly positive and the other end is slightly negative.
Ozone (O3) Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the Lewis structure of ozone, each oxygen atom has an octet of electrons, except for the central oxygen atom, which has six valence electrons.
To satisfy the octet rule, one of the lone pairs of electrons on the outer oxygen atoms can be moved to form a double bond with the central oxygen atom. This results in a resonance structure, where the double bond shifts between the outer oxygen atoms.
Ozone (O3) Bond Angles
The bond angle in ozone, O3, is approximately 116.8 degrees. This angle is less than the ideal bond angle of 120 degrees expected for a trigonal planar geometry. The deviation from the ideal angle is due to the repulsion between the lone pairs of electrons and the bonded electron pairs.
The presence of lone pairs on the central oxygen atom causes the bonded oxygen atoms to be pushed closer together, resulting in a slightly smaller bond angle than expected.
Resonance Structure of Ozone (O3)
Ozone exhibits resonance, which means that the double bond in the molecule can shift between the outer oxygen atoms. This resonance results in a more stable structure for ozone.
In the Lewis structure of ozone, the double bond can be represented between either of the outer oxygen atoms and the central oxygen atom. The resonance structure shows the delocalization of the double bond, indicating that the molecule’s electron density is spread out over the entire ozone molecule.
Geometrical Structure of Ozone (O3)
The geometrical structure of ozone can be described as bent or angular. The molecule consists of three oxygen atoms bonded together, with a bond angle of approximately 116.8 degrees. This bent shape is a result of the repulsion between the lone pairs of electrons and the bonded electron pairs.
The bent structure of ozone is important because it affects the molecule’s polarity and reactivity. The presence of a bent structure contributes to the overall polarity of the molecule.
Solved Examples on Lewis Structure of Ozone (O3)
Example 1: Draw the Lewis structure of ozone (O3) and determine its molecular geometry.
Solution:
Step 1: Calculate the total number of valence electrons in ozone. Each oxygen atom contributes six valence electrons.
Total valence electrons = 3 * 6 = 18
Step 2: Select the central atom. In the case of ozone, any one of the oxygen atoms can be chosen as the central atom.
Step 3: Connect the outer oxygen atoms to the central oxygen atom using single bonds.
Step 4: Complete the octets of the outer oxygen atoms by adding lone pairs of electrons.
Step 5: Check whether the central oxygen atom has an octet. If not, move a lone pair from an outer oxygen atom to form a double bond with the central oxygen atom.
Step 6: Check the stability of the Lewis structure by calculating the formal charge on each atom.
The molecular geometry of ozone is bent due to the repulsion between the lone pairs of electrons and the bonded electron pairs.
Example 2: Determine the hybridization of the central oxygen atom in ozone (O3).
Solution: The central oxygen atom in ozone undergoes sp2 hybridization. One 2s orbital and two 2p orbitals combine to form three sp2 hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar geometry around the central oxygen atom.
How Kunduz Can Help You Learn Lewis Structure of Ozone (O3)?
To deepen your understanding of the Lewis structure of ozone and related concepts, Kunduz offers comprehensive resources and educational materials. Through our engaging learning modules, you can explore the Lewis structure of ozone in detail and gain a solid foundation in molecular geometry, hybridization, and polarity. Kunduz is your reliable companion in the journey of mastering chemistry concepts, ensuring your academic success.
Related Topics:
