In the field of organic chemistry, hemiacetals are a class of compounds that play a significant role in various chemical reactions. These compounds are formed when an aldehyde or a ketone reacts with an alcohol. Hemiacetals are characterized by the general formula R1R2C(OH)OR, where R1 and R2 can be hydrogen or an organic substituent. The formation of hemiacetals is an essential step in the conversion of aldehydes or ketones to acetals or ketals.
What is Hemiacetal?
Hemiacetals are compounds that result from the addition of an alcohol to an aldehyde or a ketone. The addition of the alcohol group to the carbonyl carbon forms a new carbon-oxygen bond, resulting in the formation of a hemiacetal. The hemiacetal structure consists of a central carbon atom bonded to an alcohol group, an ether group, an R-group, and a hydrogen atom. The presence of both an alcohol group and an ether group makes hemiacetals unique.
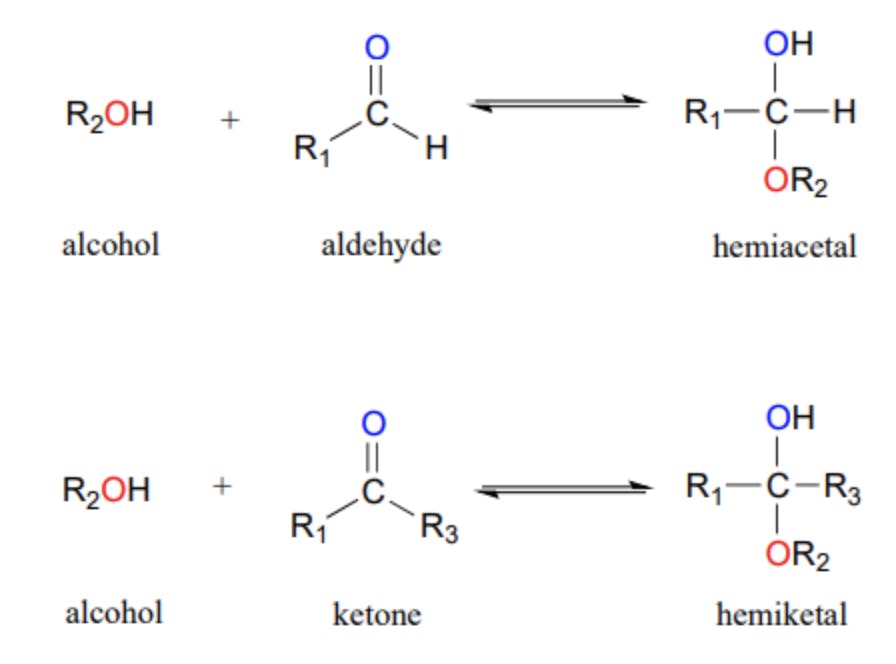
Hemiacetal Structure
The structure of a hemiacetal is characterized by a central carbon atom bonded to an alcohol group, an ether group, an R-group, and a hydrogen atom. The alcohol group (-OH) and the ether group (-OR) are attached to the same carbon atom. The R-group represents any organic substituent. The presence of the alcohol group and the ether group in the hemiacetal structure gives it distinct chemical properties.
Difference Between Hemiacetal and Acetal
Hemiacetals and acetals are closely related compounds, but they differ in their chemical structure and stability. The primary difference between hemiacetals and acetals is the number of -OR groups attached to the central carbon atom. Hemiacetals have one -OR group and one -OH group, while acetals have two -OR groups. This difference in structure gives acetals greater stability compared to hemiacetals.
To further illustrate the difference between hemiacetals and acetals, refer to the table below:
| Property | Hemiacetal | Acetal |
|---|---|---|
| Structure | One -OR group, One -OH group | Two -OR groups |
| Stability | Less stable | More stable |
| Formation | Intermediate product | Final product |
| Examples | Glucose, fructose | Polyoxymethylene, dioxolane |
Synthesis of Hemiacetal
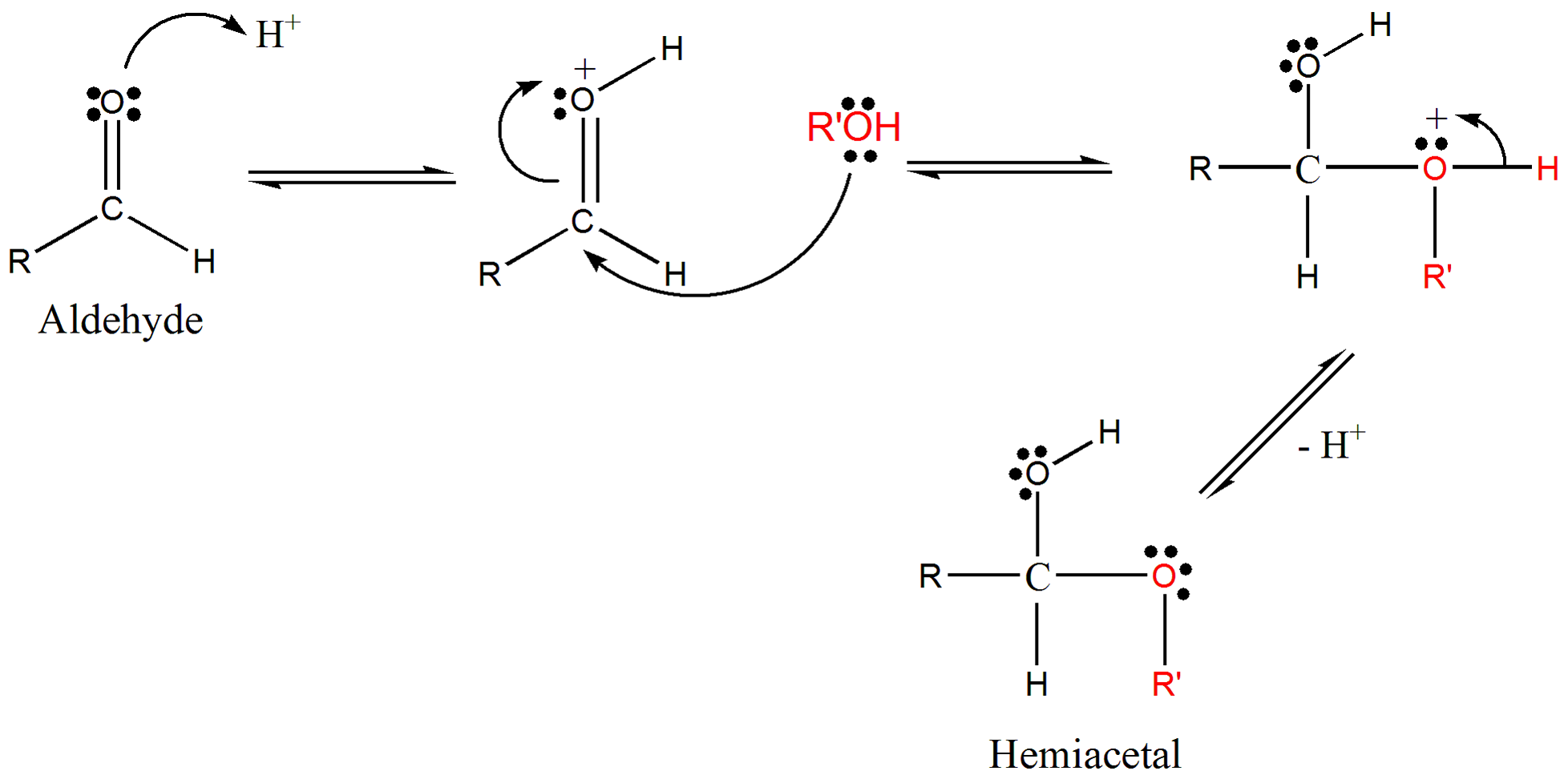
Hemiacetals can be synthesized through two methods: neutral reaction and acid-catalyzed reaction. In the neutral reaction, an aldehyde or a ketone reacts with an alcohol to form a hemiacetal. This reaction involves the alcohol attacking the carbon of the carbonyl group, resulting in the formation of a new carbon-oxygen bond.
The acid-catalyzed reaction is much faster and involves the addition of a hydrogen on the aldehyde oxygen prior to the reaction with the alcohol. This hydrogen serves as a catalyst, accelerating the formation of the hemiacetal. The acid-catalyzed reaction is commonly used in laboratory settings due to its efficiency and speed.
Hemiacetal Uses
Hemiacetals have various applications in organic synthesis and natural compounds. One of the most well-known examples of a hemiacetal is glucose. Glucose exists primarily in its cyclic hemiacetal form, where the aldehyde group and the alcohol group on the same molecule react to form a hemiacetal.
In addition to glucose, hemiacetals are also found in other sugars, such as fructose. Hemiacetals are important in the food industry as they contribute to the sweet taste of sugars. Moreover, hemiacetals are essential intermediates in the synthesis of acetals and ketals, which have numerous applications in chemistry and industry.
Hemiacetal Formation
The formation of hemiacetals occurs through the reaction between an aldehyde or a ketone and an alcohol. This reaction involves the alcohol attacking the carbonyl carbon of the aldehyde or ketone, resulting in the formation of a new carbon-oxygen bond. The presence of the alcohol group and the ether group in the hemiacetal structure makes it a crucial intermediate in the conversion to acetals and ketals.
The reaction mechanism for hemiacetal formation can be explained as follows:
- The alcohol attacks the carbonyl carbon of the aldehyde or ketone.
- The electrons from the carbon-oxygen double bond shift towards the oxygen, resulting in a negative charge on the oxygen and a positive charge on the ether group.
- The hydrogen atom from the alcohol group is transferred to the oxygen, forming a new carbon-oxygen bond and completing the formation of the hemiacetal.
What Are the Characteristics of Hemiacetal?
Hemiacetals possess several characteristic properties that distinguish them from other compounds. Here are some key characteristics of hemiacetals:
- Stability: Hemiacetals are less stable compared to acetals due to the presence of the -OH group, which can easily react with other compounds.
- Reactivity: Hemiacetals are reactive compounds and can undergo further reactions to form acetals or ketals.
- Solubility: Hemiacetals are generally soluble in polar solvents, such as water and alcohols.
- pH Sensitivity: Hemiacetals are sensitive to changes in pH and can be hydrolyzed back into their constituent compounds in acidic or basic conditions.
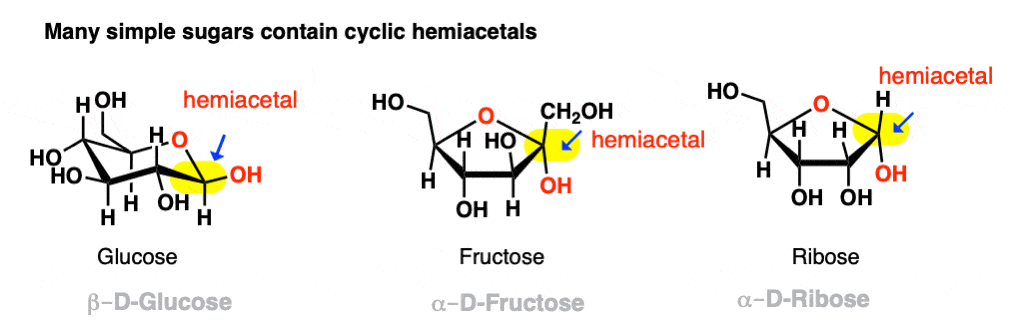
Sugars as Intramolecular Hemiacetals and Hemiketals
One of the most well-known examples of hemiacetals in nature is glucose. Glucose exists primarily in its cyclic hemiacetal form, where the aldehyde group and the alcohol group on the same molecule react to form a hemiacetal. In the case of glucose, the alcohol group on carbon 5 reacts with the aldehyde group to create a six-membered ring structure.
Fructose and other ketoses also exist as cyclic hemiketals, where the ketone group and the alcohol group on the same molecule react to form a hemiketal. The formation of these intramolecular hemiacetals and hemiketals is a result of the reaction between the carbonyl group and the hydroxyl group within the same sugar molecule.
Hemiacetals in Nature
Hemiacetals are not only found in sugars but also occur naturally in various compounds. For example, mycorrhizin A, an antibiotic found in the mycorrhizal fungus Monotropa hypopitys L., contains cyclic hemiacetal molecules. These hemiacetals play a role in the biological activity of the compound.
Additionally, hemiacetals can be found in certain pharmaceutical drugs, where they contribute to the overall structure and function of the compound. The presence of hemiacetals in natural compounds highlights their significance in biological processes and their potential applications in various fields.
Glucose Hemiacetal: Formation
The formation of the glucose hemiacetal is a crucial step in the metabolism of glucose in living organisms. Glucose exists primarily in its cyclic hemiacetal form, known as alpha-D-glucopyranose. This cyclic form is more stable than the linear form and is favored under physiological conditions.
The formation of the glucose hemiacetal occurs through an intramolecular reaction between the aldehyde group and the alcohol group. The alcohol group on carbon 5 attacks the aldehyde group on carbon 1, resulting in the formation of a six-membered ring structure. This cyclic hemiacetal form of glucose is important in its role as a source of energy in living organisms.
Solved Examples on Hemiacetal
Example 1: Consider the following reaction:
-CH3CHO + CH3OH ⇌ ?
What product is formed in this reaction?
Solution: The given reaction involves the addition of methanol to acetaldehyde. Methanol, which is an alcohol, reacts with acetaldehyde to form a hemiacetal. The product of this reaction is CH3CH(OMe)OH, where Me represents the methyl group.
Example 2: What is the difference between a hemiacetal and an acetal?
Solution: The main difference between a hemiacetal and an acetal is the number of -OR groups attached to the central carbon atom. A hemiacetal has one -OR group and one -OH group, while an acetal has two -OR groups. Acetals are more stable compared to hemiacetals due to the presence of two -OR groups, which provide greater stability to the compound.
How Kunduz Can Help You Learn Hemiacetal?
At Kunduz, we understand the importance of mastering organic chemistry concepts such as hemiacetals. Our team of experienced tutors is committed to providing comprehensive and accessible learning materials to help you succeed in your studies.
Join Kunduz today and experience the convenience and effectiveness of our learning platform. Empower yourself with the knowledge and skills needed to excel in organic chemistry and beyond.
Related Topics:
Ethane: Formula, Properties, Chemical Structure, Uses of C2H6
Carbonic Acid (C2HO3): Structure, Properties, Preparation, Uses of Carbonic Acid
Benzoic Acid (C6H5COOH): Structure, Properties, Uses of Benzoic Acid
