Inorganic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Inorganic Chemistry
P Block - Group 17A pure diatomic element is kept in a 225 mL container at 481 K and 3 670 mmHg If the mass of the sample is 1 952 grams what element is it

Inorganic Chemistry
Qualitative analysisHow much volume will 18 3 grams of carbon dioxide occupy if it s heated to 412 K at 597 Torr

Inorganic Chemistry
Coordination compoundstions a CoCl 6H O H O NH4OH NH4CI unknown compound H O b Write chemical equations for the following re c unknown compound Ag unknown compound heat CoCl

Inorganic Chemistry
Classification of Elements and PeriodicityThe melting point of a substance is the point where the vapor pressure is equal to the ambient atmospheric pressure O True O False

Inorganic Chemistry
Classification of Elements and PeriodicityA 3 00 L rigid container holds 6 26 moles of neon gas at 1 255 K What is the pressure in the container in atm

Inorganic Chemistry
Preparation and Properties of CompoundsWhat is the percent yield Answer Question 2 round to the nearest tenth nt viold in theoretical vield divided by 1 pt

Inorganic Chemistry
P Block - Group 163 34 grams of oxygen gas are stored in a 1 5 L balloon If more oxygen is added such that the volume is 9 19 L how much gas in moles was added


Inorganic Chemistry
P Block - Group 18How many grams of xenon gas will be present in a 125 mL container at 117 psi and 183 K

Inorganic Chemistry
Qualitative analysisComplete the following structure by adding additional bonds and or lone pairs of electrons OH H C C N H HH Which of the following statements is correct O There are two pi bonds in this molecule O The H N H bond angle is 120 O There are nine sigma o bonds in this molecule O The carbon atom bonded to the oxygen is sp hybridized O The hybridization of N is sp

Inorganic Chemistry
Qualitative analysisA gas at 517 K occupies 551 mL If it is cooled to 139 K what volume in mL will it occupy

Inorganic Chemistry
Classification of Elements and PeriodicityConvert 33 5 psi to bar Unit Pascal 1 N m Pounds per square inch Torr 1 mmHg Inches of mercury Bar Atmosphere Abbreviation Pa psi torr in Hg bar atm Average Air Pressure at Sea Level 101 325 Pa 14 7 psi 760 torr exact 29 92 in Hg 1 013 bar 1 00 atm

Inorganic Chemistry
Classification of Elements and PeriodicityAfter boiling mercury it is held as a monatomic gas at 49 2 kPa and 995 K If the gas occupies 0 0281 L how many grams of mercury are present

Inorganic Chemistry
Classification of Elements and PeriodicityWhich of the following statements is incorrect O Potassium K has a larger atomic radius than titanium Ti Olonization energy values are always greater than zero O Bromine Br has a higher electronegativity than iodine 1 O Electron affinity values are always greater than zero O Nitrogen N has a higher ionization

Inorganic Chemistry
Classification of Elements and PeriodicityIn 1662 Robert Boyle and an assistant carried out an experiment in which a sample of gas was subjected to a steadily increased applied pressure The results are presented in the graph below Experimental data was collected and tabulated The graph below shows the relationship between the experimental pressure and what variable Variable 10 Boyle s Gas Law Experiment 200 Pressure in mig 40 50 60 Temperature in Kelvin O Amount in moles Volume in litres O Pressure in atmospheres

Inorganic Chemistry
Classification of Elements and Periodicity3 Convert each of the following to mass in grams a 0 150 mol Ba OH 2 b 4 850 mol CCl4 c 1 355 x 1024 atoms Sr 4 Convert each of the following a 4 72 g Kr b 15 6 g He mol Kr c 12 4 mol carbon monoxide CO b 3 7 mol Na mol He 5 Calculate the following conversions a 200 0 g FeCl c 250 0 mol Al O3 g Na

Inorganic Chemistry
Preparation and Properties of CompoundsConsider the following reaction describing the combustion of propane C3H8 g 5 O g 3 CO g 4 H O g If oxygen is consumed at a rate of 20 mol L 1 s1 what is the rate of production of water 08 mol L 1 S 1 O 12 mol L S 1 O16 mol L 1 S 1 18 mol L 1 S 1 O24 mol L 1 5 1

Inorganic Chemistry
HydrogenWhich of the following only contains single bonds OHCN OH CCHCH3 OCH3CHO OCH3NH OHNNH

Inorganic Chemistry
Classification of Elements and PeriodicityWhich of the following has exactly two unpaired electrons in the ground state OF O O Ne

Inorganic Chemistry
Preparation and Properties of Compoundsincompicte Lewis structure is shown below The structure only shows the atoms and how they are connected The molecule has a net charge of zero H II Emplete the lewis structure giving all atoms full octets If there is more than one way to do this draw resonance structures showing all possibilities If not est draw one Lewis structure Be sure to write in any non zero formal charges Click and drag to start drawing a structure

Inorganic Chemistry
P Block - Group 13MISSED THIS Watch IWE 13 7 Read Section 13 8 You can click on the Review link to access the section in your e Text A 14 0 mL sample of an unknown H3PO4 solution requires 110 mL of 0 130 M KOH to completely react with the H PO4 Part A What was the concentration of the unknown H3PO4 solution H3PO4 aq 3KOH aq 3H O l K3PO4 aq VAEO S e Submit Previous Answers Request Answer M Review

Inorganic Chemistry
Qualitative analysisPhthalonitrile CsH4N2 is produced by the ammoxidation of o xylene C8H10 according to the following reaction C8H10 1 O2 g NH g C8H4N2 S H O 1 How many grams of water would be produced by the complete ammoxidation of 20 8 moles of o xylene


Inorganic Chemistry
D Block elementsThe form of a Cr III ion depends on pH and is caused by the amphoteric behavior of chromium III oxide hydroxide Demonstrate this behavior by two reactions Cr OH Cr OH 5 5p

Inorganic Chemistry
Qualitative analysisConsider the reaction between bromine gas and chlorine gas at a particular temperature Br g Cl g 2 BrCl g When the system is at equilibrium the molar concentrations of Br2 Cl and BrCl are 0 0060 M 0 0095 M and 0 015 M respectively What is the value for K for this system 0 25

Inorganic Chemistry
Preparation and Properties of Compounds20 78 The K of ZnF is 3 0x1077 at 25 C What is AG Is it possible to prepare a solution that contains Zn aq and Flag at t standard state concentrations

Inorganic Chemistry
Preparation and Properties of CompoundsIn which of the following compounds does iodine have an oxidation state of 3 OHIO3 O HOI O NalO O Nal3

Inorganic Chemistry
Classification of Elements and PeriodicityExamine the three molecules below Jose De Bur Which of the following functional groups is found in all three molecules O ether O carboxylic acid O ketone O amide ester

Inorganic Chemistry
P Block - Group 14A polymer produced after converting compound rubber to an elastic and final end use product is said to be a copolymerized b elastomeric c rubberized d vulcanized
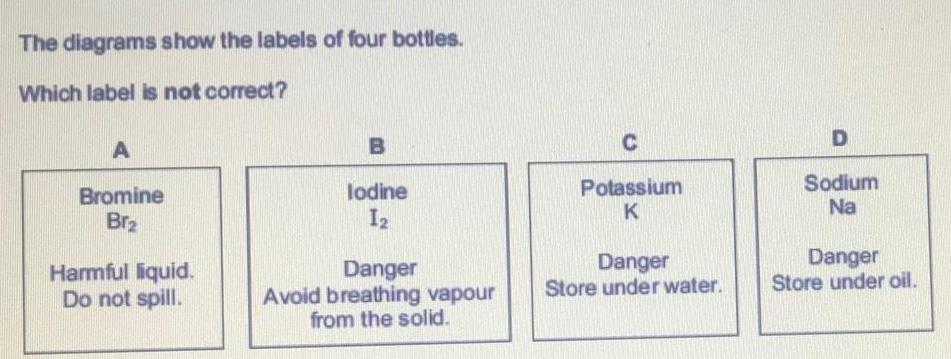
Inorganic Chemistry
S Block - Group 1The diagrams show the labels of four bottles Which label is not correct A Bromine Brz Harmful liquid Do not spill B lodine 12 Danger Avoid breathing vapour from the solid C Potassium K Danger Store under water D Sodium Na Danger Store under oil

Inorganic Chemistry
Qualitative analysisselect the correct answer Which statement best describes how Della s love for Jim affects the plot O A It makes Della want to go out for shopping to buy pretty things for herself OB It makes Della realize that she loves her own looks more than she love jim OC It motivates Della to sell her hair so that she can buy a gift for jim OD It causes Della to quit her job in order to spend more time with Jim Raset Next

Inorganic Chemistry
Preparation and Properties of CompoundsThe endothermic reaction between carbon monoxide and diiodine pentoxide is written below 5 CO g 1 05 g 12 g 5 CO2 g Which of the following would increase the amount of CO O increasing the pressure O decreasing the pressure O increasing the temperature O decreasing the temperature

Inorganic Chemistry
Classification of Elements and PeriodicityThe following set of data was obtained by the method of initial rates for the reaction H3C 3CBr OH H3C 3COH Br What is the value of the rate constant k H3C 3CBr M OH M Initial Rate M s 0 25 0 25 1 1 10 4 0 50 0 25 0 50 0 50 1 8 10 4 5 1 none of these 4 4 x 10 4 S 1 8 8 x 10 4 5 1 2 2 10 4 2 2 x 10 4

Inorganic Chemistry
Preparation and Properties of CompoundsIf 13 55 grams of HCI reacts with excess Mg at STP according to the reaction below what volume of H2 is produced Mg s 2 HCl aq H2 g MgCl2 aq

Inorganic Chemistry
HydrogenWhere does reduction occur in galvanic and electrolytic cells O at the anode in both galvanic and electrolytic cells O at the positive electrode in both galvanic and electrolytic cells O at the cathode in both galvanic and electrolytic cells O at the cathode in galvanic cells and at the anode in electrolytic cells O at the anode in galvanic cells and at the cathode in electrolytic cells

Inorganic Chemistry
D Block elementsWhich of the following ions contains 24 electrons and 27 protons Ov O Cr ON O CO A1

Inorganic Chemistry
Qualitative analysis13 The arrow points to the bond in the ATP molecule that is energy rich Name the enzyme that facilitates this adenosine P P 2001 Adenosinetriphosphate ATP name of enzyme adenosine 10

Inorganic Chemistry
Preparation and Properties of Compounds8 Place the following in order of decreasing molar entropy at 298 K A Ar N2H4 HCI C N2H4 Ar HCI B Ar HCI N2H4 D N2H4 HCI Ar

Inorganic Chemistry
Classification of Elements and PeriodicityIn which of the following series are the diatomic molecules arranged in the order of increasing bond length OF2 Cl ICI BrCl 12 OF2 Cl BrCl ICI 12 OF2 BrCl Cl I2 ICI OF2 Cl2 BrCl 12 ICI Fo IC BrCL Cla la

Inorganic Chemistry
Preparation and Properties of Compounds2 The following equation represents the reaction for formation of ammonia Sketch an enthalpy diagram for this reaction and label each axis PE and reaction coordinate as well as the reactants products activation energy and overall change in enthalpy in your diagram 8 pts H O g 2 Cl g 2 HOCI g AH 209 kJ

Inorganic Chemistry
Classification of Elements and PeriodicityEach of the following pairs contains one strong acid and one weak acid except O HNO3 and H CO3 O HF and HOCI O H SO4 and H S O CH3COOH and HCI H PO and HRr

Inorganic Chemistry
Classification of Elements and Periodicity2 How is activation energy similar to an uphill climb in a ski slope 3 What type of biological molecules are enzymes

Inorganic Chemistry
S Block - Group 2Assign oxidation numbers to all of the elements in each of the compounds or ions below 11 H SO 1 HCI 2 KNO I 3 OH 4 Mg N 5 KCIO 12 H SO 13 BaO 14 KMnO 15 LiH

Inorganic Chemistry
S Block - Group 2Beryllium nitride Be3N2 molar mass 55 056 g mol reacts with water to form beryllium hydroxide and ammonia What mass of water is required to completely consume 15 0 g of Be3N 033 7 g O 29 5 g 14 7 g 04 9 g 16g

Inorganic Chemistry
Classification of Elements and PeriodicityWhen lipid bilayers are disrupted they are able to spontaneously reseal Why

Inorganic Chemistry
Preparation and Properties of CompoundsO Calculate using stoichiometry O Divide by 100 O Multiply by 100 Convert all values to percentages Question 4 4 pts In the chemical engineering lab last week the complete decomposition of 435 8 g of ammonium dichromate molar mass 252 1 g mol produced 101 1 g H O NH4 2Cr O7 s Cr O3 s N g 4H O g 1 What is the theoretical yield of water produced Select

Inorganic Chemistry
Classification of Elements and Periodicity2 What effect does changing temperature and pH have on enzyme activity

Inorganic Chemistry
Classification of Elements and PeriodicityWhat happens to the shape of an enzyme when it is exposed to unfavorable temperature and pH

Inorganic Chemistry
Classification of Elements and PeriodicityA 2 5 L sample of unknown gas has a mass of 12 0 g and a pressure of 1 5 atm at 35 0 C What is the identity of the unknown gas Ohydrogen gas O hydrogen bromide O carbon monoxide Ochlorine gas

Inorganic Chemistry
Qualitative analysis2 When you add vinegar to water the pH level Drops b rises C drop a or rise