Inorganic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Inorganic Chemistry
Classification of Elements and PeriodicityFor the following reaction, 56.3 grams of barium hydroxide are allowed to react with 30.3 grams of sulfuric acid.
barium hydroxide (aq) + sulfuric acid(aq) --> barium sulfate(s) + water(l)
What is the maximum amount of barium sulfate that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Inorganic Chemistry
Classification of Elements and PeriodicityWhich of the following are conclusions from Rutherford's Gold Foil Experiment?
Select all that apply.
Most of the atom's volume is occupied by nearly zero-mass particles.
The nucleus is negatively charged.
The atom's mass is uniformly distributed throughout its volume.
Essentially all of the mass is concentrated in a tiny spot.
Neutral (no charge) particles are found throughout the atom's volume.

Inorganic Chemistry
Qualitative analysisThe compound sodium thiosulfate pentahydrate, Na2S2O3-5H₂O, is important commercially to the photography business as "hypo", because it has the ability to dissolve unreacted silver salts from photographic film during development. Sodium thiosulfate pentahydrate can be produced by boiling elemental sulfur in an aqueous solution of sodium sulfite.
S8 (s) + Na₂SO3(aq) + H₂O(l) → Na2S2O3.5H₂O(s) (unbalanced)
What is the theoretical yield of sodium thiosulfate pentahydrate when 2.90 g of sulfur is boiled with 14.4 g of sodium sulfite?
Theoretical yield =
Sodium thiosulfate pentahydrate is very soluble in water. What is the percent yield of the synthesis if a student doing this experiment is able to isolate (collect) only 5.20 g of the product?
Percent yield =

Inorganic Chemistry
Classification of Elements and PeriodicityIsotopes differ in the number of _ inside the nucleus and therefore they differ in _.
Select the choice that correctly fills in the blanks
protons, charge
protons, mass
electrons, charge
electrons, mass
neutrons, mass

Inorganic Chemistry
Preparation and Properties of CompoundsWhat is the change in internal energy of the system?
Express the internal energy in kilojoules to three significant figures.

Inorganic Chemistry
Qualitative analysisWhat is the enthalpy for reaction 1 reversed?
reaction 1 reversed: 2NO+ O2---> 2NO2

Inorganic Chemistry
HydrogenDetermine the enthalpy for this reaction:
2NaOH(s) + CO₂ (g)→Na2CO3 (s) + H₂O(l)

Inorganic Chemistry
Preparation and Properties of CompoundsThe molar heat capacity of silver is 25.35 J/mol · °C. How much energy would it take to raise the
temperature of 9.90 g of silver by 15.3 °C?

Inorganic Chemistry
Classification of Elements and PeriodicityCalculate ΔE, if a system releases 66.4 kJ of heat to its surroundings while the surroundings do 41.0 kJ of work on the system.
Express your answer in kilojoules using two significant figures.

Inorganic Chemistry
Preparation and Properties of CompoundsMatch the following solution with the condition identified (name precipitate for 1 extra bonus point each if there is one. If you put wrong precipitate, there is a minus one point)
a. (Answer_) K2SO4 & Na2CO3
b. (Answer_) BaCl2 & KOH
(PPT) Solid will form, Precipitation
(NX) No Precipitation, No Reaction

Inorganic Chemistry
Qualitative analysisIt takes 54.0 J to raise the temperature of an 9.90 g piece of unknown metal from 13.0°C to 24.9 °C. What is the specific heat for the metal?
Express your answer with the appropriate units.

Inorganic Chemistry
Qualitative analysisThe amount of boiling water required to raise the temperature of 25.0 kg of water in the bath to body temperature is 4.80 kg. In this process, the heat lost by the boiling water is equal to the heat gained by the room-temperature water. How much heat was transferred in this process?
Express your answer to four significant figures and include the appropriate units.

Inorganic Chemistry
Classification of Elements and PeriodicityIdentify whether each process is endothermic or exothermic:
1. the process from Part A: q = 0.768 kJ and w = -890 J ;
2. the process from Part B: a system releases 66.4 kJ of heat to its surroundings while the surroundings do 41.0 kJ of work on the system.
Both the processes from Parts A and B are endothermic.
The process from Part A is endothermic and the process from Part B is exothermic.
Both the processes from Parts A and B are exothermic.
The process from Part A is exothermic and the process from Part B is endothermic.

Inorganic Chemistry
Qualitative analysisPropane (C3H8) burns according to the following
balanced equation:
C3H8(g) +5O2(g) →3CO₂(g) + 4H₂O(g)
Calculate ΔH for this reaction using standard enthalpies of formation. (The standard enthalpy of
formation of gaseous propane is -103.9 kJ/mol.)
Express the enthalpy in kilojoules to four significant figures.
![Calculate the enthalpy change for the reaction:
using enthalpies of formation:
2 H₂O₂(l)→ 2 H₂O(l) + O₂(g)
ΔH° ƒ [H₂O2] = −187.8 kJ/mol
ΔH° ƒ [H₂O] = -285.8 kJ/mol
-98.0 kJ
-196.0 kJ
+98.0 kJ
+196.0 kJ
more information needed](https://media.kunduz.com/media/sug-question/raw/57555062-1659725045.8898091.jpeg?w=256)
Inorganic Chemistry
Classification of Elements and PeriodicityCalculate the enthalpy change for the reaction:
using enthalpies of formation:
2 H₂O₂(l)→ 2 H₂O(l) + O₂(g)
ΔH° ƒ [H₂O2] = −187.8 kJ/mol
ΔH° ƒ [H₂O] = -285.8 kJ/mol
-98.0 kJ
-196.0 kJ
+98.0 kJ
+196.0 kJ
more information needed

Inorganic Chemistry
Qualitative analysisA total of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 189 g of solution. The reaction caused the temperature of the solution to rise from 21.00 to 24.70 °C. What is the enthalpy of this reaction? Assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water.
Enter your answer in kilojoules per mole of compound to three significant figures.

Inorganic Chemistry
Qualitative analysisWhen a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water,
the temperature rose from 23.9 C to 32.0 C. Calculate ΔH in kJ/mol NaOH) for the solution
process:
NaOH(s) --> Na+ (aq) + OH-(aq)
Assume it's a perfect calorimeter and that the specific heat of the solution is the same as that of
pure water.

Inorganic Chemistry
Qualitative analysisThe amount of space an object occupies may be found by
h. mass
f. length
g. time
i. volume
Which of the following measurements is most precise?
a. 5.432
C. 5.4
d. 5
b. 5.43

Inorganic Chemistry
Classification of Elements and Periodicity(1) Identify each of the following half-reactions as either an oxidation half-reaction or a reduction half-reaction.
Cr³+ (aq) + 3e- ---> Cr(s)
Mn(s)→ Mn2+ (aq) + 2e-
(2) Write a balanced equation for the overall redox reaction. Use smallest possible integer coefficients.

Inorganic Chemistry
S Block - Group 2Select all the statements that are true when magnesium reacts with chlorine:
A. The compound that forms is MgCl
B. Magnesium transfers electrons to chlorine
C. Magnesium becomes a +2 cation
D. Chlorine becomes a -2 anion
DE. Chlorine transfers electrons to magnesium
F. The formula for the compound formed is MgCl2
G. Chlorine becomes an anion
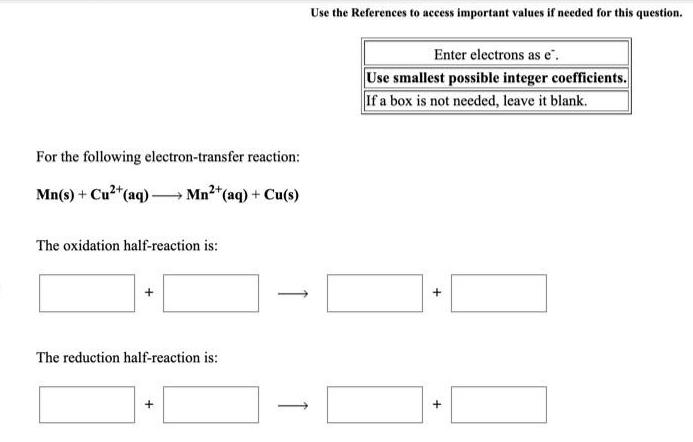
Inorganic Chemistry
Preparation and Properties of CompoundsFor the following electron-transfer reaction:
Mn(s) + Cu²+ (aq) → Mn²+ (aq) + Cu(s)
The oxidation half-reaction is:
The reduction half-reaction is:
Use the References to access important values if needed for this question.
Enter electrons as e-.
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.

Inorganic Chemistry
Classification of Elements and PeriodicityWhen the following equation is balanced properly under basic conditions, what are the coefficients of the species shown?
F- + Br₂-> Br- + F2
Water appears in the balanced equation as a
Which element is reduced?
(reactant, product, neither) with a coefficient of
(Enter 0 for neither.)

Inorganic Chemistry
Classification of Elements and PeriodicityA raindrop has a mass of 50. mg and the Pacific Ocean has a mass of 7.08 × 10^20 kg.
Use this information to answer the questions below. Be sure your answers have the correct number of significant digits.
What is the mass of 1 mole of raindrops?
Round your answer to 2 significant digits.
How many moles of raindrops are in the Pacific Ocean?
Round your answer to 2 significant digits.

Inorganic Chemistry
Preparation and Properties of CompoundsA 14.0 g sample of an aqueous solution of hydrobromic acid contains an unknown amount of the acid.
If 29.0 mL of 7.88×10-² M barium hydroxide are required to neutralize the hydrobromic acid, what is the percent by mass of hydrobromic acid in the mixture?
% by mass

Inorganic Chemistry
Qualitative analysisYou have three known solutions:
1) Na₂CO3
2) BaCl2
3) KNO3
Even though you know that you have these three solutions, you do not know which one is which because they
are not labeled. Which one of the following reagents should you add to each solution to be able to accurately
identify and label them?
Select one:
A. We need more than one of these reagents to be able to identify all three solutions.
B. CaCl2
C. H₂SO4
D. HCI
E. NaOH

Inorganic Chemistry
Preparation and Properties of CompoundsIdentify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction.
3F2+2Al- →6F- +2A1³+
species oxidized
oxidizing agent
species reduced
reducing agent
As the reaction proceeds, electrons are transferred from _ to _

Inorganic Chemistry
Preparation and Properties of CompoundsThe gasoline in an automobile gas tank has a mass of 60.0 kg and a density of 0.752 g/mL
A) What's the specific gravity
B) Calculate the volume of gasoline in gallons (Show your calculations):

Inorganic Chemistry
Preparation and Properties of CompoundsSnO₂²- + 2NH₂OH = SnO3²- + N₂H4+ H₂O
In the above reaction, the oxidation state of tin changes from
How many electrons are transferred in the reaction?

Inorganic Chemistry
Preparation and Properties of CompoundsWhat volume of 2.5 M Nitric acid (HNO3) is required to prepare 500 mL of a 0.20 M HNO3 solution
40 mL
0.2 mL
500 mL
2.5 mL

Inorganic Chemistry
Preparation and Properties of CompoundsDoes a reaction occur when aqueous solutions of iron(II) iodide and lead(II) nitrate are combined?
no yes
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.

Inorganic Chemistry
Preparation and Properties of CompoundsWhen the following skeletal equation is balanced under basic conditions, what are the coefficients of the species shown?
I2 + Cl- ---> I- + Cl2
Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.)
Which species is the oxidizing agent?

Inorganic Chemistry
Preparation and Properties of CompoundsIron(III) sulfide reacts with HCI (g) to produce iron(III) chloride and hydrogen sulfide. When 56.8 g of iron(III) sulfide reacts with 42.3 g of hydrochloric acid to produce 54.2 g of iron(III) chloride, what is the percent yield for
the reaction?
Molar masses:
Fe (55.85 g/mol)
S (32.07 g/mol)
Cl (35.45 g/mol)
H (1.008 g/mol)
Do NOT include units in your answer. If you round during your calculation, be sure to keep at least three (3) decimal places. Report your answer to one (1) decimal place.

Inorganic Chemistry
MetallurgyWhen the following half reaction is balanced under basic conditions, what are the coefficients of the species shown?
NiO₂ + H₂O ---> Ni(OH)₂ + OH-
In the above half reaction, the oxidation state of nickel changes from _ to _

Inorganic Chemistry
Preparation and Properties of CompoundsWhen the following half reaction is balanced under acidic conditions, what are the coefficients of the species shown?
Mn2+ H₂O ---> MnO₂ + H+
In the above half reaction, the oxidation state of manganese changes from _ to _

Inorganic Chemistry
Preparation and Properties of Compoundsto access important values if needed for this question.
When the following skeletal equation is balanced under basic conditions, what are the coefficients of the species shown?
Br₂ NH₂OH = N2H4+ Br2
Water appears in the balanced equation as a0 for neither.)
Which species is the reducing agent?

Inorganic Chemistry
Preparation and Properties of CompoundsA solution contains 35 g of KBr dissolvedin 205 g of water. Express the
concentration of the solution as %
(m/m).
14.6 % (m/m)
12.3 % (m/m)
5.86 % (m/m)
17.1 % (m/m)

Inorganic Chemistry
Qualitative analysisA 14.9 g sample of an aqueous solution of hydrochloric acid contains an unknown amount of the acid. If 16.0 mL of 1.03 M sodium hydroxide are required to neutralize the hydrochloric acid, what is the percent by mass of hydrochloric acid in the mixture? % by mass

Inorganic Chemistry
Preparation and Properties of CompoundsYou are exposed to a small amount of
radiation daily. This small radiation
exposure is the
background radiation
relative biological efficiency
personal radiation
inherent radiation

Inorganic Chemistry
Preparation and Properties of CompoundsAn unknown compound has the following chemical formula:
P₂Ox
where x stands for a whole number.
Measurements also show that a certain sample of the unknown compound contains 6.3 mol of oxygen and 2.50 mol of phosphorus.
Write the complete chemical formula for the unknown compound.

Inorganic Chemistry
Classification of Elements and PeriodicityHow much energy in kilocalories is lost when a sample 225 mL water cools from 75.0°C to
22.0°C? (Specific Heat Capacity of water is 1.00 cal/g °C or 4.184 J/g°C) Density 1.00 g/mL
(Show your calculations

Inorganic Chemistry
Classification of Elements and PeriodicityWhat volume of a 0.170 M barium hydroxide solution is required to neutralize 27.3 mL of a 0.141 M perchloric acid solution?
mL barium hydroxide

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the mass percent of element A in a compound with molecular
formula A2B3. The molar mass of element A is 35.5, and molar mass of element B is
48.5. When input answer, convert percentage to decimal. Use 3 decimal places. For
example, if you answer is 56.7%, convert it to 0.567

Inorganic Chemistry
Preparation and Properties of CompoundsThe movie below shows some molecules in a tiny sample of a mixture of gases.
Drag the slider to see how the molecules move.
Does a chemical reaction happen during this
movie?
If a chemical reaction does happen, write a
balanced chemical equation for it.

Inorganic Chemistry
Preparation and Properties of CompoundsMagnesium metal, Mg (s), reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen gas. When 43.5 g of magnesium is added to 258 mL of 6.00 M hydrochloric acid, what mass (in grams) of hydrogen is produced, assuming the reaction goes to completion?
Molar masses:
Mg (24.31 g/mol)
CI (35.45 g/mol)
H (1.008 g/mol)
Do NOT include units in your answer. If you round during your calculation, be sure to keep at least three (3) decimal places. Report your answer to one (1) decimal places.

Inorganic Chemistry
Qualitative analysisA 1.45-g sample of phosphorus burns in air and forms
2.57 g of a phosphorus oxide. Calculate the empirical for-
mula of the oxide. (Hint: Determine the mass of oxygen in
the 2.57 g of phosphorus oxide by determining the differ-
ence in mass before and after the phosphorus burns in air.)

Inorganic Chemistry
Classification of Elements and PeriodicitySolid iron and aqueous magnesium chloride are produced by the reaction of aqueous iron(III) chloride and solid magnesium. Write a balanced chemical equation for this reaction

Inorganic Chemistry
Qualitative analysisA chemistry student weighs out 0.215 g of phosphoric acid (H₂PO4), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1500 M NaOH solution.
Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.

Inorganic Chemistry
Classification of Elements and Periodicity0.29 g sodium iodide reacts with chlorine, producing 0.075 g sodium chloride and some amount of iodine. Find the percent yield of NaCl. Write and balance the equation first. Don't forget diatomics and how to write formulas.

Inorganic Chemistry
Classification of Elements and Periodicity2.0 liters of ethanol (C₂H6O, density = 0.789 g/mL) combusts with 2.0 kilograms oxygen gas to produce carbon dioxide and water. What is the maximum mass of water than can form? Write the balance equation first.

Inorganic Chemistry
Classification of Elements and PeriodicitySilver ion reacts with chloride ion to form the precipitate silver chloride:
Ag+(aq)+C1-(aq) AgCl(s)
After the reaction reached equilibrium, the chemist filtered 99% of the solid silver chloride fromthe solution, hoping to shift the equilibrium to the right, to form more product. Critique the chemist's experiment.