Preparation and Properties of Compounds Questions and Answers

Inorganic Chemistry
Preparation and Properties of CompoundsA student adds 6.50 g of dry ice (solid CO2) to an empty balloon. What will be the volume of the balloon at STP after all the dry ice sublimes (converts to gaseous CO₂)?

Inorganic Chemistry
Preparation and Properties of CompoundsA sample of oxygen gas is collected over water at 25°C and a total pressure of 652 torr. The volume of the gas collected is 500.0 mL. What mass of oxygen is collected? The vapor pressure of water at 25°C is 23.8 torr.

Inorganic Chemistry
Preparation and Properties of CompoundsA 3.94 gram sample of an unknown gas is found to occupy a volume of 2.48 L at a pressure of 610 mm Hg and a temperature of 56 °C. The molar mass of the unknown gas is __ g/mol.

Inorganic Chemistry
Preparation and Properties of CompoundsWhat volume is occupied by a 1.66 mol sample of krypton gas at a temperature of 0 °C and a pressure of 1 atm?

Inorganic Chemistry
Preparation and Properties of CompoundsA sample of neon gas at a pressure of 0.875 atm and a temperature of 23.3 °C, occupies a volume of 15.0 liters. If the gas is allowed to expand at constant temperature to a volume of 19.2 liters, the pressure of the gas sample will be ___ atm.

Inorganic Chemistry
Preparation and Properties of CompoundsA sample of krypton gas collected at a pressure of 456 mm Hg and a temperature of 298 K has a mass of 114 grams. The volume of the sample is____L.

Inorganic Chemistry
Preparation and Properties of CompoundsThe average lung capacity of a human is 6.0 L. How many moles of air are in your lungs when you are in the following situations?
Assume air behaves ideally and has an average molar mass of 29.0 g/mol.
a. At sea level (T = 298 K, P = 1.00 atm).
b. 10 m below water (T = 298 K, P = 1.98 atm).
c. At the top of Mount Everest (T= 203 K, P = 0.297 atm).

Inorganic Chemistry
Preparation and Properties of CompoundsA 0.7011-g mixture of sodium chloride and sodium nitrate is completely dissolved in water. When silver nitrate is added to the solution, 0.9805 g of solid precipitate. What is the percent by mass of chloride in the initial mixture?
34.59%
71.50%
65.41 %
28.50 %
13.99 %

Inorganic Chemistry
Preparation and Properties of CompoundsNitrogen monoxide is a pollutant commonly found in smokestack emissions. One way to remove it is to react it with ammonia.
4NH3(g) + 6NO(g) → 5N₂(g) + 6H₂O(l)
How many liters of ammonia are required to change 49.6 L of nitrogen monoxide to nitrogen gas? Assume 100% yield and that all gases are measured at the same temperature and pressure.

Inorganic Chemistry
Preparation and Properties of CompoundsA compound has the empirical formula CHI. A 256-mL flask, at 373 K and 750. torr, contains 2.31 g of the gaseous compound. Give the molecular formula.

Inorganic Chemistry
Preparation and Properties of CompoundsA 1.25 mol sample of neon gas occupies a volume of _____L at STP.

Inorganic Chemistry
Preparation and Properties of CompoundsEnter the electron configuration for the ion most likely formed by phosphorus.

Inorganic Chemistry
Preparation and Properties of CompoundsComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column.

Inorganic Chemistry
Preparation and Properties of CompoundsSucrose (C12H22011) is combusted in air according to the following reaction:
C12H22O11(s) + O₂(g) → CO₂(g) + H₂O(l)
How many moles of carbon dioxide would be produced by the complete combustion of 70.3 grams of sucrose in the presence of excess oxygen?

Inorganic Chemistry
Preparation and Properties of CompoundsWhat does it mean to say that a solution is saturated with a solute?
It contains the maximum amount of solute possible at a particular temperature and is in equilibrium with undissolved solute.
It contains the minimum amount of solute possible at a particular temperature and is in equilibrium with undissolved solute.
It contains the maximum amount of solute possible at a particular temperature and is in equilibrium with dissolved solute.
It contains the minimum amount of solute possible at a particular temperature and is in equilibrium with dissolved solute.

Inorganic Chemistry
Preparation and Properties of CompoundsHydrogen cyanide (HCN) is a poisonous gas that can be produced in the lab by reacting propane (C3H₂) with NH3 to produce HCN and H₂ (all in the gaseous state).
How many grams of ammonia are required to produce 14.4 g of HCN if the reaction runs to 65% completion?
Lets solve this question in a series of steps:
When properly balanced the coefficients for each species in the reaction are:

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the mass of oxygen in the copper oxide. The molar mass of copper is 63.55 g/mol and the molar mass of oxygen is 16.00 g/mol.

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the pKa values for the acids shown below and then rank them in order of increasing acidity (weakest to strongest). (b) If the concentration of each acid is 0.10 M, which acid if any will produce the highest concentration of H3O*? Where applicable, show your work and report the pKa's to two decimal places. (5 pts)
Acid 1: Kal = 2.20 x10-7 Acid 2: Ka2 = 2.20 x10-8 Acid 3: Ka3 = 1.00 x10-7

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the enthalpy of the reaction
4B (s) + 302 (g) -> 2B₂O3(s)
given the following pertinent information:
1. B2O3 (s) + 3H₂O(g)→302 (g) + B₂ H6 (g), ΔH = +2035 kJ
2. 2B(s) + 3H₂(g) →B₂ H6 (g), ΔH = +36 kJ
3. H₂(g) + O2(g) →H₂O(l), ΔH = -285 kJ
4. H₂O(1)→H₂O(g), ΔH = +44 kJ
Express your answer with the appropriate units.

Inorganic Chemistry
Preparation and Properties of CompoundsYou may want to reference (Page) Section 12.2
while completing this problem. The following soluble salts are strong electrolytes. For each, enter a balanced equation for their dissociation in water.
Part B
NaNO3
Express your answer as a chemical equation. Identify all of the phases in your answer.
A chemical reaction does not occur for this question.
,

Inorganic Chemistry
Preparation and Properties of CompoundsIdentify which sets of quantum numbers are valid for an electron. Each set is ordered (n, l, me, m.).
4,2,1,1/2
3,-2,-2,-1/2
3,2,-1,-1/2
1,2,0,-1/2
1,1,0,-1/2
3,-2,-1,0
3,2,0,1/2
3,0,0,1/2
2,1,0,1/2
1,3,0,1/2
4,3,4,-1/2
0,2,1,-1/2
![Give the ground-state electron configuration for copper (Cu) using noble-gas shorthand.
Express your answer in condensed form as a series of orbitals. For example, [Ar]4s²3d³ would be entered as [Ar]4s^23d^8.](https://media.kunduz.com/media/sug-question/raw/58389947-1659730076.4336758.jpeg?w=256)
Inorganic Chemistry
Preparation and Properties of CompoundsGive the ground-state electron configuration for copper (Cu) using noble-gas shorthand.
Express your answer in condensed form as a series of orbitals. For example, [Ar]4s²3d³ would be entered as [Ar]4s^23d^8.

Inorganic Chemistry
Preparation and Properties of CompoundsFor which of the following reactions is AHxn equal to AH of the product(s)? You do not need to look up
any values to answer this question.
Check all that apply.
CO(g) + 1/2O2(g) →CO₂(g)
2Na(s) + F2 (g)→2NaF(s)
CaCO3(g)→CaO+CO2(g)
C(s, graphite) + O2(g) →CO₂(g)
Na(s) + F2(1)→NaF (s)
Na(s)+F2 (g) →NaF (s)

Inorganic Chemistry
Preparation and Properties of CompoundsConsider the following three moving objects:
i. A golf ball with a mass of 45.9 g moving at a speed of 50.0 m/s.
ii. An electron moving at a speed of 3.5 × 105 m/s.
iii. A neutron moving at a speed of 2.3 x 10² m/s.
List the three objects in order from shortest to longest de Broglie wavelength.
iii<i<ii
i<ii<iii
iii<ii<i
ii<iii<i
i<iii<ii

Inorganic Chemistry
Preparation and Properties of CompoundsPart B
Green light has a frequency of about 6.00 x 10¹4 s-¹. What is the energy of a photon of green light?
Express your answer to three significant figures and include the appropriate units.
Ephoton=

Inorganic Chemistry
Preparation and Properties of CompoundsThe solubility of silver sulfate in water is 5.05 grams per liter.
If a silver sulfate solution had a concentration of 5.05 grams per liter, it would be said to be
saturated
supersaturated
unsaturated

Inorganic Chemistry
Preparation and Properties of CompoundsPart A
A microwave oven operates at 3.00 GHz. What is the wavelength of the radiation produced by this appliance?
Express the wavelength numerically in nanometers.

Inorganic Chemistry
Preparation and Properties of CompoundsThe titration of a 20.0-mL sample of an H₂SO4 solution of unknown concentration requires 22.87 mL of a 0.158 M KOH solution to reach the equivalence point. What is the concentration of the unknown H₂SO4 solution?

Inorganic Chemistry
Preparation and Properties of CompoundsWhich of the following evidence does not support Alfred Wegener's idea of continental drift?
(A)Fossils of plants and animals in climates where their survival would have been impossible.
(B)The thickness of layers of ice in the Antarctic.
(C)Mountain ranges on different continents lined up when coastlines were matched up.
(D)Glacier scrapings on rocks on different continents lined up when coastlines were matched up.

Inorganic Chemistry
Preparation and Properties of CompoundsFor each bond, show the direction of polarity by selecting the correct partial charges.
I-F
I-CI
F-Cl
The most polar bond is

Inorganic Chemistry
Preparation and Properties of CompoundsWhat volume of hydrogen gas is produced when 56.1 g of sodium reacts completely according to the following reaction at 25 °C and 1 atm?
sodium (s) + water (l) →sodium hydroxide (aq) + hydrogen(g)

Inorganic Chemistry
Preparation and Properties of CompoundsIn a certain city the homicide rate is decreasing at the rate of 3% per year. What is the exact half-life of the crime rate, rounded to two decimal places?

Inorganic Chemistry
Preparation and Properties of CompoundsA sample of hydrogen gas at 302 K and 0.376 atm occupies a volume of 3.57 L. If the gas is compressed-into a smaller volume, while at the same time it is heated to a higher temperature, the final gas pressure
could be higher or lower than 0.376 atm depending on the final volume and temperature.
will be lower than 0.376 atm.
will be higher than 0.376 atm.

Inorganic Chemistry
Preparation and Properties of CompoundsA 0.424 gram sample of hydrogen gas has a volume of 907 milliliters at a pressure of 3.29 atm. The temperature of the H₂ gas sample is

Inorganic Chemistry
Preparation and Properties of CompoundsA chemist prepares a solution of silver(II) oxide (AgO) by measuring out 0.0031 μmol of silver(II) oxide into a 400. ml. volumetric flask and filling the flask to the mark with water.
Calculate the concentration in mmol/L of the chemist's silver(II) oxide solution. Round your answer to 2 significant digits.

Inorganic Chemistry
Preparation and Properties of Compounds0.776 mol sample of hydrogen gas at a temperature of 21.0 °C is found to occupy a volume of 25.4 liters. The pressure of this gas sample is

Inorganic Chemistry
Preparation and Properties of CompoundsWrite a 150 words for your initial response. Your first response is due by midnight of
the second day of the unit. Provide links to the resources you used to conduct your research.
You must respond to at least two other classmates on a separate day by midnight of the last
day of the unit for full credit. While you can be supportive of your peers by saying, "I agree" or
"Wow, I never thought of that", these statements alone are not sufficient, you must explain
why. Any posts made after the last day of the unit will not be considered for a grade.
Answer the question(s) below:
During preparation for an MODL amalgam on tooth #4, the dentist notices that the decay
extends well beyond his initial assessment and is very close to the pulpal chamber. He
informs the patient that the treatment plan must be altered to see how the tooth will react
now that the decay has been removed. What changes will need to be made in the setup?

Inorganic Chemistry
Preparation and Properties of CompoundsAlthough we generally think of combustion reactions as involving oxygen gas, other rapid oxidation reactions are also referred to as combustions. For example, if magnesium metal is placed into chlorine gas, a rapid oxidation takes place, and magnesium chloride is produced.
Mg(s) + Cl₂(g) → MgCl2 (s)
What volume of chlorine gas, measured at STP, is required to react completely with 4.34 g of magnesium?

Inorganic Chemistry
Preparation and Properties of CompoundsA gas made up of homonuclear diatomic molecules escapes through a pinhole 0.859 times as fast as N₂ gas. Write the chemical formula of the gas.

Inorganic Chemistry
Preparation and Properties of CompoundsThe half-life, T, for a particular radioactive element is 12 min. Find the decay rate of the element.

Inorganic Chemistry
Preparation and Properties of CompoundsCalculate the number of milliliters of 0.636 M NaOH required to precipitate all of the Mg2+ ions in 187 mL of 0.718 M Mg(NO3)2 solution as Mg(OH)2. The equation for the reaction is:
Mg(NO3)2(aq) + 2NaOH(aq) → Mg(OH)₂(s) + 2NaNO3(aq)

Inorganic Chemistry
Preparation and Properties of CompoundsDetermine the oxidation state for each of the elements below.
The oxidation state of
The oxidation state of
The oxidation state of
chlorine in
cadmium in
ices to access important value
oxygen in
chlorate ion
CIO3
cadmium hydroxide
Cd(OH)2
potassium peroxide
K₂O₂
IS
is
is

Inorganic Chemistry
Preparation and Properties of CompoundsFor the following reaction, 19.8 grams of carbon dioxide are allowed to react with 38.6 grams of potassium hydroxide.
carbon dioxide (g) + potassium hydroxide (aq)-potassium carbonate (aq) + water (l)
What is the maximum amount of potassium carbonate that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Inorganic Chemistry
Preparation and Properties of CompoundsWhat volume of a 0.181 M potassium hydroxide solution is required to neutralize 18.8 mL of a 0.167 M hydrobromic acid solution?

Inorganic Chemistry
Preparation and Properties of CompoundsWhat is the change in internal energy of the system?
Express the internal energy in kilojoules to three significant figures.

Inorganic Chemistry
Preparation and Properties of CompoundsThe molar heat capacity of silver is 25.35 J/mol · °C. How much energy would it take to raise the
temperature of 9.90 g of silver by 15.3 °C?

Inorganic Chemistry
Preparation and Properties of CompoundsMatch the following solution with the condition identified (name precipitate for 1 extra bonus point each if there is one. If you put wrong precipitate, there is a minus one point)
a. (Answer_) K2SO4 & Na2CO3
b. (Answer_) BaCl2 & KOH
(PPT) Solid will form, Precipitation
(NX) No Precipitation, No Reaction
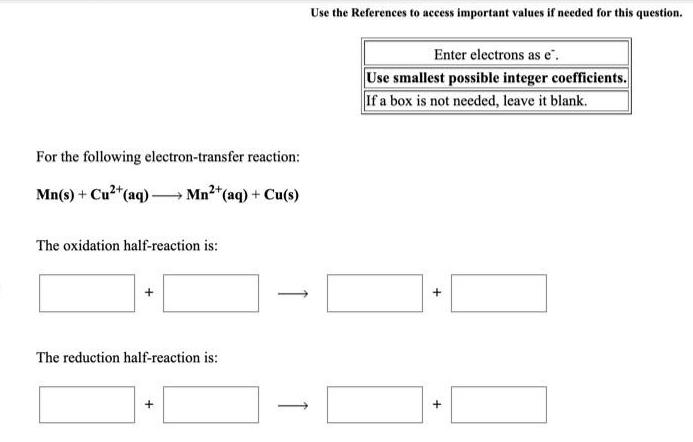
Inorganic Chemistry
Preparation and Properties of CompoundsFor the following electron-transfer reaction:
Mn(s) + Cu²+ (aq) → Mn²+ (aq) + Cu(s)
The oxidation half-reaction is:
The reduction half-reaction is:
Use the References to access important values if needed for this question.
Enter electrons as e-.
Use smallest possible integer coefficients.
If a box is not needed, leave it blank.

Inorganic Chemistry
Preparation and Properties of CompoundsA 14.0 g sample of an aqueous solution of hydrobromic acid contains an unknown amount of the acid.
If 29.0 mL of 7.88×10-² M barium hydroxide are required to neutralize the hydrobromic acid, what is the percent by mass of hydrobromic acid in the mixture?
% by mass

Inorganic Chemistry
Preparation and Properties of CompoundsIdentify the species oxidized, the species reduced, the oxidizing agent and the reducing agent in the following electron transfer reaction.
3F2+2Al- →6F- +2A1³+
species oxidized
oxidizing agent
species reduced
reducing agent
As the reaction proceeds, electrons are transferred from _ to _