Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
Biomolecules7- The best definition of a prosthetic group
is that:
a) It is a long chain of poly peptide bond
b) It is the complete amino acid sequence of
a protein
c) It is the secondary structure of a protein
d) It is the portion of the protein that does
not contain amino acid
e) None of the above

Organic Chemistry
General organic chemistryWhich of these would be considered an example of vertical integration?
Standard Oil controlling 90% of the oil industry
The Industrial Workers of the World (IWW) being a union that was open to all workers, even unskilled immigrants
Political machines providing immigrants with jobs in exchange for votes
Carnegie Steel owning the mines that produced the iron it would make into steel, as well as owning the boats that brought the iron to the steel mills

Organic Chemistry
General organic chemistryFor the following reaction, 6.09 grams of perchloric acid (HCIO4) are mixed with excess tetraphosphorus decaoxide.
The reaction yields 1.86 grams of phosphoric acid.
perchloric acid (HClO4) (aq) + tetraphosphorus decaoxide (s)-phosphoric acid (aq) + dichlorine heptaoxide (1)
What is the theoretical yield of phosphoric acid ?
What is the percent yield of phosphoric acid ?

Organic Chemistry
General organic chemistry2C6H5 Cl + C₂ HOCl3 → C14H9 Cl5 + H₂O
In a government lab, 1167 g of chlorobenzene is reacted with 486 g of chloral.
a. What mass of DDT is formed, assuming 100% yield?
b. Which reactant is limiting? Which is in excess?
Mass
.
c. What mass of the excess reactant is left over?
d. If the actual yield of DDT is 288.0 g, what is the percent yield?
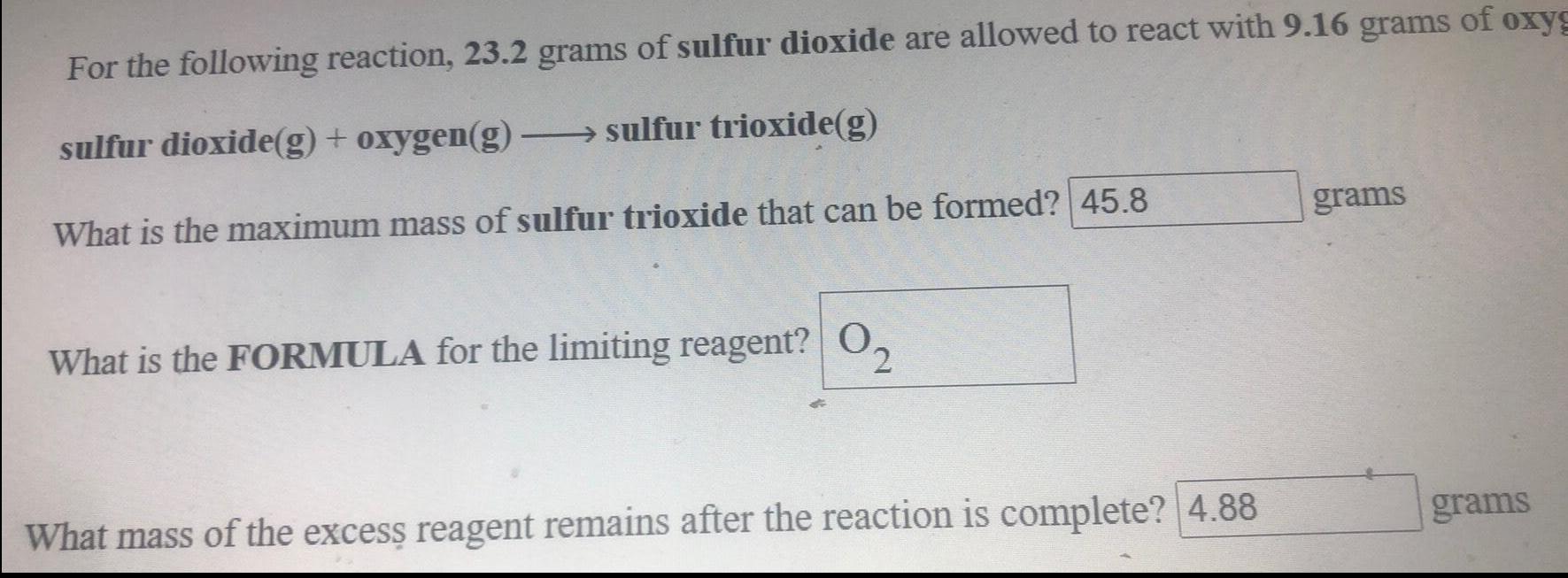
Organic Chemistry
General organic chemistryFor the following reaction, 23.2 grams of sulfur dioxide are allowed to react with 9.16 grams of oxygen
sulfur dioxide(g) + oxygen(g) →→→sulfur trioxide(g)
What is the maximum mass of sulfur trioxide that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryFor the following reaction, 9.93 grams of nitrogen monoxide are allowed to react with 8.91 grams of oxygen gas.
nitrogen monoxide(g) + oxygen(g) → nitrogen dioxide(g)
What is the maximum mass of nitrogen dioxide that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryDraw the expanded structural formula for 1,3-dichlorocyclopentane. An expanded structural formula shows all the atoms of the molecule and all the
bonds between the atoms in the molecule.

Organic Chemistry
General organic chemistryConsider the common arrow types used in organic chemistry listed below. Ordered list
A. nucleophilic attack by a single atom
B. heterolytic o bond cleavage
C. nucleophilic attack by a w bond
D. bond dissociation
E. bond formation
Which of these types are the reverse of each other?

Organic Chemistry
EthersWhat direction (d or I; + or -) would you expect the enantiomer of naproxen to rotate plane-polarized light?
(d). (-)
(d), (+)
(l). (+)
(l). (-)

Organic Chemistry
Chemistry in Daily LifeFour Fe²+ ions are key components of hemoglobin, the protein that transports oxygen in the blood. If you assume that these ions are 4Fe²+, how many
protons and neutrons are present in each nucleus, and how many electrons are present in each ion?
protons
neutrons
electrons

Organic Chemistry
Practical DetectionGive the name for each of the following compounds, which contain a polyatomic ions
a. CuHPO4:
b. LiClO₂:
c. Pb(SO4)2:

Organic Chemistry
HydrocarbonsWhat is the name of the compound with the formula MgSO3 ?
What is the name of the compound with the formula KNO₂ ?

Organic Chemistry
General organic chemistryWhat is the name of the compound with the formula CO₂?
What is the name of the compound with the formula NF3 ?
What is the name of the compound with the formula N₂O ?

Organic Chemistry
General organic chemistryWhich of the following statements is valid?
2-butene; enantiomers
2,4-dimethylhexane; superimposable mirror images
2-bromobutane;
achiral molecule
propene; center of asymmetry.

Organic Chemistry
General organic chemistryClassify the following as a heterogeneous mixture, homogeneous mixture (solution), or a pure substance: hot coffee with sugar (completely dissolved)
A. heterogeneous mixture
B. homogeneous mixture/solution
C. pure substance

Organic Chemistry
HydrocarbonsThe structure below belongs to the class of organic compounds called.......and it's IUPAC name is CH3CH2CH2CCCH2CH3
A. Alkane; Dehydrogenated heptane
B. Alkene; 4- Heptene
C. Alkyne; 4- Heptyne
D. Alkyne; 3- Heptyne
E. Alkene; Hexene

Organic Chemistry
General organic chemistryThe equation below represents the acid catalyzed ____ of ____ to form
USE IUPAC NAMES ONLY
CH2=CH-CH3 + H₂=====> CH3CH(OH)CH3
A. carboxylation; propyne; 1-propanol
B. reduction; 2-propene; 1-propanol
C. hydration; propene; 2-propanol
D. hydration; propane; dimathylether
E. hydrogenation; propene; 2-propanol

Organic Chemistry
Chemistry in Daily LifeWhat is the name of the compound with the formula NH₂HCO3 ?
What is the name of the compound with the formula NH NO₂ ?
What is the name of the compound with the formula (NH4)3PO4?

Organic Chemistry
General organic chemistryEthanol, CH3CH₂OH, has a pKa value of 15.9 while acetic acid, CH3COOH, has a pKa value of 4.74. This data indicates that
ethanol is a stronger acid than acetic acid.
the conjugate base of ethanol is a stronger base than that of acetic acid.
the conjugate base of ethanol would not react with acetic acid.
the ratio of acetic acid to its conjugate base is larger than that of ethanol

Organic Chemistry
General organic chemistryWhich of the following objects contains at least one plane of symmetry?
Hint 1. How to determine planes of symmetry
wooden chair
work glove
a pair of scissors
metal screw

Organic Chemistry
PolymersWhen solid red phosphorus, P4, is burned in air, the phosphorus combines with oxygen, producing a choking cloud of tetraphosphorus decoxide. Choose the unbalanced chemical equation for this reaction.
P3O2 (9)→ P4 (s) + O2(g)
P4(s) + N2₂ (9)→ P4N10 (9)
P4 (s) + O2(g) → P4O10 (9)
P4(s) + H₂O(1)→ P₂O10 (9)

Organic Chemistry
General organic chemistryYou were running GC experiments on your unknown as well as unknown + sample spikes. Your unknown had two components, but when you got to the last sample containing a "spike,"your syringe became clogged. Since you were in a hurry, you quickly borrowed a syringe from the neighboring instrument; however, you ended up with four peaks in your chromatogram! What is the easiest and fastest thing to do to finish your lab?
Re-mix all of your samples and run all the chromatograms again.
Re-run your last sample after rinsing the syringe with that sample.
Run the sample on a different GC.
Answers A and B
Answers A and C
Answers B and C

Organic Chemistry
IsomerismLiquid octane (CH₂(CH₂)6CH3) reacts with gaseous oxygen gas (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water
(H₂O). If 102. g of carbon dioxide is produced from the reaction of 78.81 g of octane and 165,6 g of oxygen gas, calculate the percent yield of carbon dioxide.
Round your answer to 3 significant figures.
and

Organic Chemistry
General organic chemistryWhich of the following is NOT an important consideration in planning a recrystallization of compound X in solvent Y:
the solubility of X in solvent Y at the boiling point of Y
the boiling point of Y
the solubility of X in solvent Y at 0 °C h
the molecular weight of X
the solubility of known impurities in solvent Y

Organic Chemistry
Halogen Derivatives2-Bromobutane reacts faster than 2-chlorobutane in both the Nal/acetone test and the AgNO3/EtOH test because____
bromine is a smaller atom than chlorine, allowing for a faster reaction
the conjugate of chloride has a lower pka than the conjugate of bromide
chlorine is more electronegative than bromine
bromine is a better leaving group

Organic Chemistry
General organic chemistryWe saw in lab that although iodine is a much bigger atom than chlorine, iodine has a much lower conformational energy cost than
chlorine as an axial substituent.
What best explains this phenomenon?
The very long carbon-iodine bond minimizes steric strain.
The very long carbon-iodine bond minimizes torsional strain.
The outermost electrons of iodine are much more dispersed and therefore strain energy is reduced.
Olodine's heavier atomic weight allows it to reside further away from the chair structure.

Organic Chemistry
General organic chemistryIn this lab you synthesized Methyl acetoacetate via a Claisen Condensation reaction, which is combining two carbonyl containing molecules in the presence of a base. What functional group(s) were present in these two carbonyl reactants needed for this reaction?
Two esters
Two aldehydes
An ester and a ketone
Two ketones

Organic Chemistry
General organic chemistryA gaseous system that is 160.0 K and with a pressure of 126.6 kPa, has its temperature changed to 267.6 K. What is the new pressure of the system? Do not include units in your answer, they are presumed to be kPa. Round your answer to the nearest hundredth.

Organic Chemistry
General organic chemistryWhich of the following helps us to predict the effect of change in temperature on a reaction system?
Select the correct answer below:
Collision theory
The ideal gas law
Le Châtelier's principle
The effects of changes in temperature on equilibrium reactions are entirely random and cannot be predicted.

Organic Chemistry
EthersDraw the following (in any format) and write the molecular formula:
4) 3-iodo-2-methylhexano-
5) 1,2-dichloroethane
6) 2-chloro-4-ethylnonane
7) 4-isopropyldecane
8) 2,2,4-tribromo-3-fluorooctane
9) 1,1-difluoro-3-methylcyclohexane
10) 1-ethylcyclobutane

Organic Chemistry
General organic chemistryWhich statement about solutions is true?
Select the correct answer below:
The solute must be the same physical state as the solvent.
A solution can have only one solute.
A solution can have many solutes.
Given enough time, the solute will separate from the solvent.

Organic Chemistry
General organic chemistryConsider the reaction below. According to the Bronsted-Lowry definition, what acts as
the conjugate acid when the reaction proceeds in the forward direction?
CN + H₂O = OH + HCN
Select the correct answer below:
CN
OOH
OHCN
H₂O

Organic Chemistry
General organic chemistryA solution of a compound in water conducts electricity, turns litmus red, and has a sour taste. What compound might be in the solution?
Select the correct answer below:
NaHCO3
Mg(OH)2
C3H5(COOH)3
NH3

Organic Chemistry
BiomoleculesCarboxypeptidase A is an enzyme that cleaves on the amino side of any C-terminal amino acids except arginine, lysine, or proline. What would be the correct peptide fragments formed if Val-Ile-His-Thr-Arg was treated with
Carboxypeptidase A?
Val lle His Thr Arg
Val lle His Thr-Arg
Val-lle-His-Thr Arg
Val-lle His-Thr Arg

Organic Chemistry
General organic chemistryWhich of the following phenomona is characteristic of unstable nuclei as opposed to stable nuclei?
Select the correct answer below:
Radioactivity
Multiple isotopes
Chemical reactivity
Atomic masses (in amu) unequal to the mass number of the isotope.

Organic Chemistry
IsomerismAlkanes:
1) Draw the skeletal/line formula for octane, cyclopropane, and pentane.
2) Write the molecular formula for octane, cyclopropane, and pentane.
3) Draw as many isomers as you can with the formula C6H12. Name all the structures.

Organic Chemistry
General organic chemistryWhich of the following two compounds is a stronger base: Sodium hydrosulfide (NaSH) or sodium hydroxide (NaOH)? In 1-2 complete and concise sentences, explain your logic in making this assignment of base strength.

Organic Chemistry
Hydrocarbons13. Select the statement that is most true regarding kinetic and thermodynamic
enolates.
a. kinetic enolates are formed with a higher energy of activation under
irreversible conditions relative to thermodynamic enolates.
b. kinetic enolates are formed with a higher energy of activation under
reversible conditions relative to thermodynamic enolates.
c. kinetic enolates are formed with a lower energy of activation under
reversible conditions relative to thermodynamic enolates.
d. kinetic enolates are formed with a lower energy of activation under
irreversible conditions relative to thermodynamic enolates.

Organic Chemistry
BiomoleculesThe composition of triglycerides is best described by which of the following statements?
Triglycerides are composed of two unsaturated fatty acids and one saturated fatty acid linked to a glycerol backbone via éster bonds.
All of these describe possible triglycerides.
Triglycerides are composed of three saturated fatty acids linked to a glycerol backbone via ester bonds.
Triglycerides are composed of three unsaturated fatty acids linked to a glycerol backbone via ester bonds.
Triglycerides are composed of two saturated fatty acids and one unsaturated fatty acid linked to a glycerol backbone via ester bonds.

Organic Chemistry
General organic chemistryAn ester of formula C7H1402 gives an alcohol and an acid when hydrolyzed. When the alcohol is
isolated and oxidized, it forms a ketone. Which of these compounds could be the ester?
b. sec-butyl propanoate
d. tert-butyl propanoate
a. isopropyl propanoate
c. ethyl 2,2-dimethylpropanoate

Organic Chemistry
General organic chemistryFor a certain reaction K is known to be 0.003. The reaction quotient was found to be
0.003
Which way will the reaction proceed?
towards the products
not enough info to know
it is at equilbrium
towards the reactants

Organic Chemistry
General organic chemistryWhat is the average rate of production of carbon dioxide for the system between 2.0 and 4.0 minutes, CH4(g)+2O2(g)->CO2(g)+2H₂O(g), if the concentration of carbon dioxide is 2.5 mol/L after 2.0 minutes and 7.2 mol/L after 4.0 minutes?
2.35 mol/(L'min)
1.18 mol/(L*min)
42.6 mol/(L*min)
0.426 mol/(L* min)
0.952 mol/(L' min)

Organic Chemistry
General organic chemistryKeep the test open while you are doing this question on a piece of paper. After you
finish the test, submit a picture of your answer in the dropbox within 5 minutes of
finishing
A mixture of 9 moles of A, 10 moles of B, and 27 moles of C is placed in a one-liter
container at a certain temperature. The reaction is allowed to reach equilibrium. The equilibrium
constant K for the reaction is 10.
A (g) + 2 B (g) <==> 3 C (g) + 5 D (s)
a) Write the general equilibrium constant equation for this reaction
b) Calculate Q.
c) Will the reaction proceed left or right and why

Organic Chemistry
General organic chemistryWhich one of the following ionic solids would be expected to have the largest lattice energy?
AIP (aluminium phosphide)
MgS (Magnesium sulfide)
NaCl (Sodium chloride)
KCI (Potassium chloride)
RbCl (Rubidium chloride)

Organic Chemistry
General organic chemistryWhich of the following is most likely a heavier stable nucleus?
Select all that apply:
A nucleus with a neutron:proton ratio of 1.05
A nucleus with a neutron:proton ratio of 1.49
The nucleus of Sb-123
A nucleus with a mass of 187 and an atomic number of 75

Organic Chemistry
General organic chemistryA concentrated weak acid is best described as which of the following?
A solution with a low pH
A solution where the concentration of undissociated acid particles is low
compared to the concentration of hydronium ions
A solution where the concentration of hydronium ions is large compared to the
concentration of undissociated acid particles
A solution with a high pH
A solution where the concentration of undissociated acid particles is high and
the relative quantity of hydronium ions is small
![K is 7.7x10-15 for the reaction 2CO(g)=C(s) + CO2(g) At a certain time the following
concentrations are measured.
[CO]=0.034M
[CO2]=3.6x10-17
Which way will the reaction proceed?
not enough info to know
towards the products
towards the reactants
it is at equilbrium](https://media.kunduz.com/media/sug-question/raw/53142838-1659042473.5846057.jpeg?w=256)
Organic Chemistry
General organic chemistryK is 7.7x10-15 for the reaction 2CO(g)=C(s) + CO2(g) At a certain time the following
concentrations are measured.
[CO]=0.034M
[CO2]=3.6x10-17
Which way will the reaction proceed?
not enough info to know
towards the products
towards the reactants
it is at equilbrium

Organic Chemistry
General organic chemistryWhat product(s) are expected in the ethoxide-promoted ß-elimination reaction of 1-chloro-1-methylcyclohexane? Omit ions, salts, and ethanol from your response.
![Consider this unbalanced equilibrium: N2(g) + H2(g) <====> NH3(g) + 94 kJ The equilibrium law expression for the balanced chemical equation would be:.
[N₂][H₂]/[NH3]
[NH3]²/[H₂][N₂]
[NH3] / [H₂][N2]
[NH3]²/[H₂]³[N₂]
2[NH3]2/3[H₂]³[N₂]](https://media.kunduz.com/media/sug-question/raw/53142918-1659010498.5152102.jpeg?w=256)
Organic Chemistry
General organic chemistryConsider this unbalanced equilibrium: N2(g) + H2(g) <====> NH3(g) + 94 kJ The equilibrium law expression for the balanced chemical equation would be:.
[N₂][H₂]/[NH3]
[NH3]²/[H₂][N₂]
[NH3] / [H₂][N2]
[NH3]²/[H₂]³[N₂]
2[NH3]2/3[H₂]³[N₂]

Organic Chemistry
General organic chemistryWhich of the following statements is TRUE regarding chemical equilibrium?
chemical equilibrium only apply to solutions
chemical equilibrium only apply to gases
increasing the temperature in an exothermic reaction shifts the equilibrium towards the side of the reactants
at equilibrium, the rate of reaction from reactants to products and the reverse is zero