Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
General organic chemistryRank the following molecules based on decreasing boiling point (highest boiling point to lowest boiling point).
I>II>III > IV
II>I>III > IV
I>III>IV > II
IV>III>I> II
IV> III>II>I

Organic Chemistry
General organic chemistryAt the gym, a man pulls a bar on a machine that works the muscles of the upper back. It takes him 0.5 seconds to raise 30 kilograms of weights a vertical distance of 0.5 meters.
Which of these exerts the same power output? (Estimate g as 10 m/s2.)
A Alifting 25 kilograms a distance of 2.4 meters in 2.0 seconds
B lifting 45 kilograms a distance of 2.4 meters in 3.0 seconds
C leg pressing 45 kilograms a distance of 0.5 meters in 0.5 seconds
D bench pressing 30 kilograms a distance of 0.5 meter in 1.5 seconds

Organic Chemistry
Chemistry in Daily LifeWhich of the following best illustrates the theory of delegate representation?
I always vote with my my fellow party members.
"I vote for what's best, even if I think the people I represent my not agree."
"My job is to represent the people who elected me regardless of my point of view."
Whether I vote the way my constituents prefer or the way I think is best depends on the issue.

Organic Chemistry
HydrocarbonsRank the following starting materials in order of increasing rate of reaction (slowest to fastest) in an
SN1 reaction:
3<1<2
1<2<3
2<3<1
1<3<2
2<1<3

Organic Chemistry
HydrocarbonsProvide the products and mechanisms for reactions a) and b). For c) provide the products only necessary to complete the synthesis. Indicate what mechanism was used for each product.

Organic Chemistry
General organic chemistryAt equilibrium, the total amount of the product(s)
is always greater than the total amount of the reactan
is always less than the total amount of the reactants
is always equal to the total amount of the reactants
may be equal to, greater than, or less than the total am

Organic Chemistry
General organic chemistryIron (III) metal is placed in a container filled with chlorine gas. When the equation is balanced, what is the coefficient for chlorine?
a) 3
b) 1
c) 4
d) 2

Organic Chemistry
General organic chemistryConsider the following equation: 6Li + N₂ → 2Li3N
a. If 21.48 mol of lithium react with 12.8 mol of nitrogen, which is the limiting reactant? (1)
b. How many moles of lithium nitride can be produced? (1)
c. How many moles of the excess reactant are left over? (1)

Organic Chemistry
Practical DetectionChlorobenzene, C6H5Cl, is used in the production of chemicals such as aspirin and dyes. One
way that chlorobenzene is prepared is by reacting benzene, C6H6, with chlorine gas according to the following BALANCED equation.
C6H6 (l) + Cl₂ (g) --------- 6H5Cl (s) + HCl (g)
12.01 X 6
a. If the theoretical yield for C6H5Cl is 65.2 g and the actual yield is 63.7 g, what is the percent yield? Insert photo of work here:

Organic Chemistry
General organic chemistryFind the partial pressure of carbon dioxide in a gas mixture with a total pressure of 30.5 kPa if the partial pressures of the other two gases in the mixture are 16.5 kPa and 3.7 kPa.
Show work below:

Organic Chemistry
Chemistry in Daily LifeConsider the following reaction: 4 FeS2 + 11 O2 → 2 Fe2O3 + 8 SO2
When 26.62 moles of FeS2 reacts with 5.44 moles of O2, how many moles of SO2 are formed?
What is the limiting reactant?
For the reactant in excess, how many moles are left over at the end of the reaction

Organic Chemistry
Chemistry in Daily LifeWhat is stoichiometry used for?
A. To determine the amount of a substance
B. To determine the atoms in a formula
C. To determine the shape of a molecule
D. To determine the molar mass of a molecule

Organic Chemistry
HydrocarbonsPropose the structure of the following C10H12O2
IR band: peak at 1720 cm-¹
¹H NMR: triplet, 2.53 ppm, 2H
triplet, 2.85 ppm, 2H
singlet, 3.60 ppm, 3H
singlet, 7.19ppm, 1H
doublet, 7.23 ppm, 2H
doublet, 7.28 ppm, 2H

Organic Chemistry
Reactions of benzeneProvide the structure of the major organic product after steps: 1), 2), 4) in the reaction sequence.
1. NaNO2, HCl, cold
2. CuCN
3. CH3CH₂CH₂MgBr
4. H3O+

Organic Chemistry
General organic chemistryAll of the processes you'll see in this assessment takes place in liquid chloroform, CHCl3. Draw a full Lewis structure for chloroform, showing all atoms, bonds, and lone pairs. Also annotate your structure with δ+ and δ- signs for partial charges.

Organic Chemistry
General organic chemistryIn each of the following reactions, assign oxidation states to each of the atoms to identify the substance that is oxidized (loses electrons) and the substance that is reduced (gains electrons).
a) Cr₂O3 + 2 Al → Al₂O3 + 2 Cr
b) 3 H₂+ Fe₂O3 → 2 Fe + 3 H₂O
c) 6 Ag + 3 HS- + 2 CrO4+ 5H₂O → 3 Ag₂S + 2 Cr(OH)3 + 7 OH-

Organic Chemistry
Chemistry in Daily Life1.98 g of calcium chloride and 3.75 g of sodium oxide are combined. Theoretically, what mass of solid product could be formed from these amounts of reactants? What is the limiting reactant?

Organic Chemistry
General organic chemistryFor an exothermic reaction that is thermodynamically favorable which of the following must be true?
A) ΔG must be positive
B) ΔS must be positive
C) ΔS must be negative
D) None of the above must be true

Organic Chemistry
Practical DetectionThe equilibrium constant for the following reaction is 650 at 298K.
CH3OH(l) CO(g) + 2H₂(g)
The value of ΔG for this reaction is _______ kJ/mol
A) 0
B) some number less than zero
C) some number greater than zero
D) Equal to K

Organic Chemistry
General organic chemistryFor a the following reaction ΔH = 6.2 kJ and ΔS = -33 J/K. Under what temperature and condition is the reaction spontaneous?
A) WhenT< -210K
B) WhenT< 210K
C) The reaction is not spontaneous at any temperature.
D) WhenT> -210K

Organic Chemistry
General organic chemistryAspirin (C9H8O4) if formed from the reaction of salicylic acid (C7H603) with liquid acetic acid according to the equation below. After the reaction the water is evaporated and solid aspirin is collected. How many grams of aspirin could be prepared by mixing 25.0 ml of 0.10 M C₂H603 with 5.00 ml of acetic acid (d=1.05 g/ml)
C7H6O3(aq) + HC2H3O2(l)→ C9H8O4(aq) + H₂O(l)

Organic Chemistry
Chemistry in Daily LifePipes can get clogged due to formation of precipitates that build up in the pipes. Examples of precipitate deposits that can cause clogged pipes include magnesium and calcium oxides that result from hard water formation. Assume that calcium chloride and sodium oxide are the starting substances (reactants) in the reaction:
a. Write a balanced chemical equation describing the formation of the "hard water" precipitation process, using the information in the case study.
b. State the names of the products that are produced from the reaction that you wrote in part (a).
c. What type of reaction(s) is/are being represented by the chemical reaction you wrote in part (a)?

Organic Chemistry
HydrocarbonsRank the substrates below in order of increasing reactivity (from slowest to fastest) in a substitution reaction with KCN in DMSO. (Tos = tosylate)
1<3<2
3<2<1
2<3<1
3<1<2
1<2<3

Organic Chemistry
General organic chemistryNew concept cars in the Detroit Auto Show feature engines that burn hydrogen gas in air to produce water vapor. Suppose that fuel tank contains 150. L of H: gas at 20.0 atm pressure at 25 °C. If all of this gas is burned, what mass of water vapor is produced?
Write a balanced chemical equation for the reaction.
Given: Unknown:
Show work below:

Organic Chemistry
Chemistry in Daily LifeK3N(s) + 2 H₂(g) KNH₂(s) + 2 KH(s) AH = -192 kJ/mol
Which of the following is the most likely reason that the reaction occurs at a significant rate only if the temperature of the reaction mixture is greater than 200°C?
A) The reaction is exothermic.
B) ΔS for the reaction is negative.
C) The reaction has a high activation energy.
D) ΔG is negative at temperatures less than 200°C.

Organic Chemistry
General organic chemistryThe following successive ionization energies correspond to an element in the third row of the periodic table:
I1 = 786.3 kJ/mol,
I2=1,580 kJ/mol,
I3= 3,230 kJ/mol,
I4=4,360 kJ/mol,
I5 = 16,000 kJ/mol, and
I6=20,000 kJ/mol.
Based on this pattern of ionization energies, identify the element.
A. Mg
B. Al
C. Si
D. P

Organic Chemistry
General organic chemistryDetermine the volume of oxygen gas (in L) needed to completely combust 0.202 L of butane gas (C4H10) at constant temperature and pressure according to the following equation:
2C4H10(g) + 13O2(g) -> 8CO2(g) + 10H₂O(l)

Organic Chemistry
Practical DetectionWhat would happen to the pressure inside a sealed syringe if the temperature was increased
from 500 K to 1000 K?
The gas inside the syringe would liquify
The pressure would increase
The pressure would decrease
The pressure would remain the same

Organic Chemistry
Chemistry in Daily LifePredict and justify the sign of ΔS° for the reaction:
HgO(s) ⇒ Hg(l) + ¹/2 O₂(g) ΔG = 128 kJ/mol
A) ΔS is positive because AG is positive
B) ΔS is negative because AG is positive
C) ΔS is positive because the products have more moles of gas than the reactants
D) ΔS is negative because the products have more moles of gas than the reactants

Organic Chemistry
General organic chemistryAnswer the following questions for the reaction below at 298K
C₂H₂(g) + 4F2(g) <-> 2CF4(g) + H₂(g) ΔH = -93.3 kJ
a) Predict the sign of the entropy change, ΔS for the reaction. Explain the basis for your prediction.
b) If the temperature at which the reaction occurs is increased to 500K, the entropy, ΔS at is -358 J/K.
i) Calculate ΔG and explain if the reaction is spontaneous at this temperature.
ii) Calculate the equilibrium constant, Keq at this temperature.
iii) Is the K value you calculated consistent with the value of ΔG for the reaction? Explain.

Organic Chemistry
Chemistry in Daily LifeWhen the hydrate CaSO4 . 2 H₂O(s) is heated the reaction below occurs.
CaSO4 . 2 H₂O(s) CaSO4(s) + 2 H₂O(g)
a) Using the data in the table below, calculate the value of AG, in kJ/mol, for the reaction at 298 K.
Substance ΔGt (kJ/mol)
CaSO4 . 2 H₂O(s) -1775
CaSO4(g) -1310
H₂O(g) -221
b) Using the value you calculated for AG, would this reaction be thermodynamically favorable?
Given that the value of AH° for the reaction at 298 K is +105 kJ/mol, calculate the value of ΔS° for the reaction at 298 K. Include units with your answer.

Organic Chemistry
General organic chemistryRank the following molecules in order from least soluble in water to most soluble in water.
Cyclohexanol, 2-decanol, methanol

Organic Chemistry
General organic chemistryFor the reaction energy diagram shown below, which of the following statements is true?
A catalyst could make the forward reaction more exergonic.
According to the Hammond postulate, B resembles C in structure more than B resembles A in structure.
The equilibrium constant for the forward reaction is greater than 1.
The reaction mechanism has four elementary steps.
The reverse reaction is endergonic.

Organic Chemistry
Chemistry in Daily LifeThe most common source of copper (Cu) is the mineral chalcopyrite (CuFeS₂). How many kilograms of chalcopyrite must be mined to obtain 480. g of pure Cu?
Express your answer numerically in kilograms.

Organic Chemistry
BiomoleculesNaCl has a molar mass of 58 g/mol. How would you prepare a 0.5 M aqueous solution of NaCl?
Add 58 grams of salt to 100 g of water
Add 29 g of salt to make 1 liter of solution
Add 58 g of salt to make 1 liter of solution
Add 29 grams of salt to 1 liter of water

Organic Chemistry
Chemistry in Daily LifeIsotope ?-35 has an atomic mass of 35. 75% of all atoms of element? are of this form. Isotope ?-37 has an atomic mass of 37. 25% of all atoms of element ? are of this form. What is the relative atomic mass of the element?

Organic Chemistry
General organic chemistryDraw all staggered and eclipsed Newman projections of the compound below rotating a full 360°. Calculate the energy of each conformation using the given values. To save space when drawing, you may abbreviate the t- butyl group as TB. Circle the conformation(s) with the highest energy. You may start with the conformation drawn below (15 pts).

Organic Chemistry
BiomoleculesDraw skeletal structures of the following:
a. cis-mono-unsaturated fatty acid with 14 carbons
b. trans-mono-unsaturated datty acid with 16 carbons
c. tri-unsaturated fatty acid with 18 carbons (your choice of trans or combination for this question)
-Are trans fats bad for you and explain your answer?

Organic Chemistry
General organic chemistrySynthesis of a compound in an achiral environment which has enantiomers results in a racemic mixture. Which of the following accounts for this observation?
Enantiomeric transition state
Magnitude of the rate constants
Magnitude of the activation energies
All of these factors are involved.

Organic Chemistry
Chemistry in Daily LifeHydrogen sulfide is an impurity in natural gas that must be removed. One common removal method is called the Claus process, which relies on the reaction: 8H₂S(g) + 402(g)→S8 (1) +8H₂O(g) Under optimal conditions the Claus process gives 98% yield of Sg from H₂S.
Part A
If you started with 45.0 grams of H₂S and 40.0 grams of O₂, how many grams of Sg would be produced, assuming 98 % yield?

Organic Chemistry
Chemistry in Daily LifeAutomotive air bags inflate when sodium azide, NaN3, rapidly decomposes to its component elements: 2NaN3 (s)-2Na(s) + 3N2 (g)
How many moles of N₂ are produced by the decomposition of 1.30 mol of NaN3?

Organic Chemistry
Practical DetectionWhat volume (mL) of 0.0829 M phosphoric acid can be neutralized with 144 mL of 0.361 M sodium hydroxide?
1880
12.9
627
1600
209

Organic Chemistry
General organic chemistryWhat is a crystal? Recrystallization is a powerful technique for purification used ubiquitously in organic chemistry. It can allow a chemist to take an amorphous, impure lump of crude product and transform it into a pile of shiny, virtually contaminant-free crystals of the target sample. What are the best ways to grow pure, contaminant-free crystals? Describe the steps of recrystallization from dissolution to nucleation and finally to growth. What is the visual difference between a crystalline, pure sample and an impure crystal? What other physical properties differ between pure and impure solid samples? Write a short essay describing how recrystallization is used in the lab, how best to execute a recrystallization and answering these questions. Please cite and include all sources for your information, including pages from your lab manual/textbook.

Organic Chemistry
Practical DetectionPropenoic acid, C3 H4 O2, is a reactive organic liquid that is used in the manufacturing of plastics, coatings, and adhesives. An unlabeled container is thought to contain this liquid. A 0.270-g sample is combusted to produce 0.172 g of water and 0.208 g of carbon dioxide.
Calculate the experimental mass % of C and H in the unknown liquid used in the experiment. Express your answers, separated by commas, to three significant figures.


Organic Chemistry
General organic chemistryWhen do we use Simple Past tense? Check ALL correct answers.
A) a feeling in the past
B) an action or event that was in progress
C) an action or event that was finished in the past
D) an action that started in the past and still continues
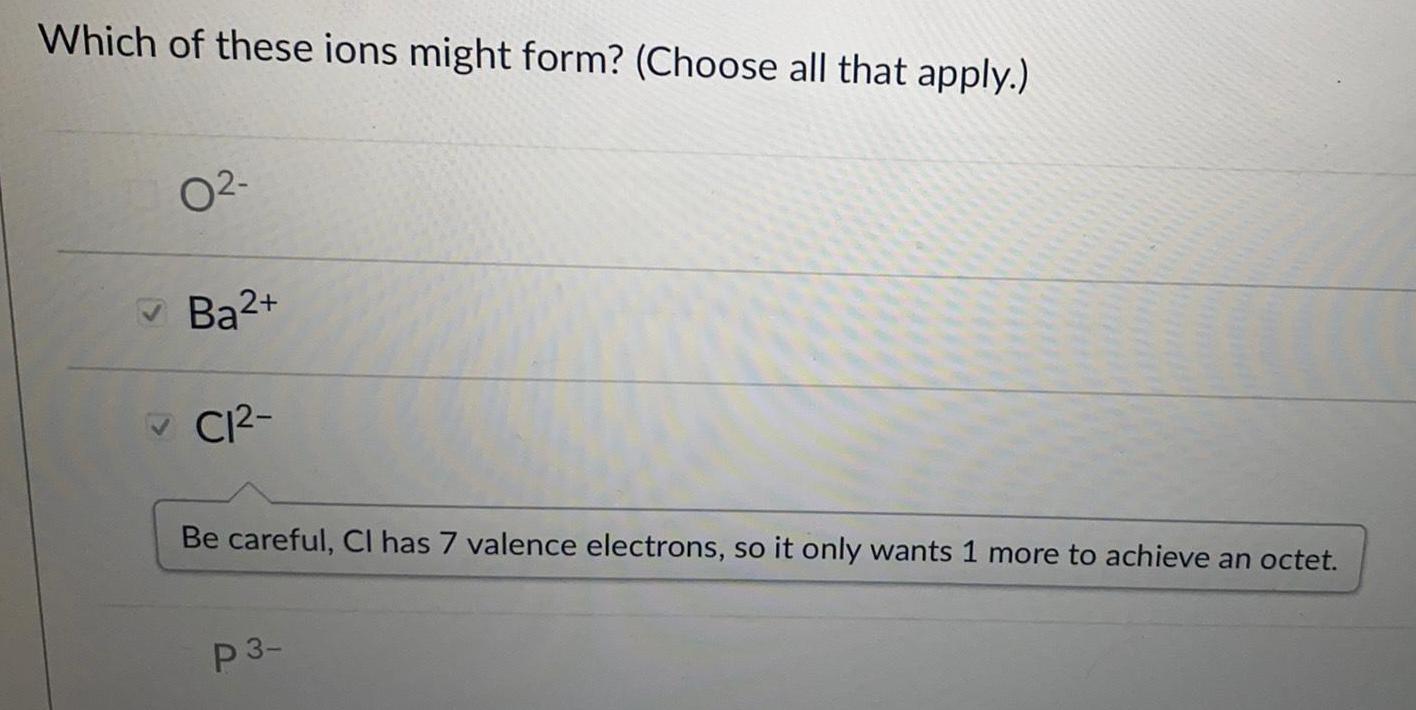

Organic Chemistry
General organic chemistryCheck the sentence with the CORRECT use of a stative verb.
A) Are you wanting to have more children now?
B) Do you want to have more children now?
C) Are you want to have more children now?

Organic Chemistry
General organic chemistrya. Convert phosphoenolpyruvate, an intermediate in glucose metabolism, to a skeletal structure.
b. Draw all reasonable resonance structures that have a phosphorus atom surrounded by 10 electrons.
c. Draw all reasonable resonance structures in which all second- and third-row atoms have an octet.

Organic Chemistry
General organic chemistryWhat does the following sentence mean (look at the main verb)?
"In recent years, Thayer has been talking to groups around the world."
A) A few years ago, she talked to other travelers.
B) She is talking to groups now.
C) She started talking to groups not a long time ago, and she still does it.
D) She does not talk to groups anymore.